Paveway™ PLUS Microbial Expression System for the Efficient Production of Therapeutic Proteins

Paveway™ is an innovative expression system in Escherichia coli (E. coli) that enables the creation of novel recombinant strains where the lead strain selection is based on titer possible within 6 weeks of program initiation. Since its launch in 2007, Paveway has become a well proven E. coli expression system, producing >140 different therapeutic proteins successfully expressed with a variety of accumulation routes including intracellular soluble, intracellular insoluble and periplasmic secretion. In addition to the potential for reduced cost of goods, Paveway also makes possible antibiotic-free cGMP production, without compromising yields and versatility.
Our Paveway™PLUS advanced workflow launched in 2022 enables strain selection based on both product titer and quality, identifying the lead strain within 11 weeks of program initiation.
Better by Design
The platform is based on a set of unique protein expression plasmids which have been developed by FUJIFILM Diosynth Biotechnologies (Figure 1). These plasmids use a number of powerful promoters, such as λpL, tac and T7A3, to enable the use of a large range of E. coli hosts. The inclusion of a stability enhancement system in the plasmids based on the cer recombination site eliminates the need for antibiotics to assure plasmid retention.
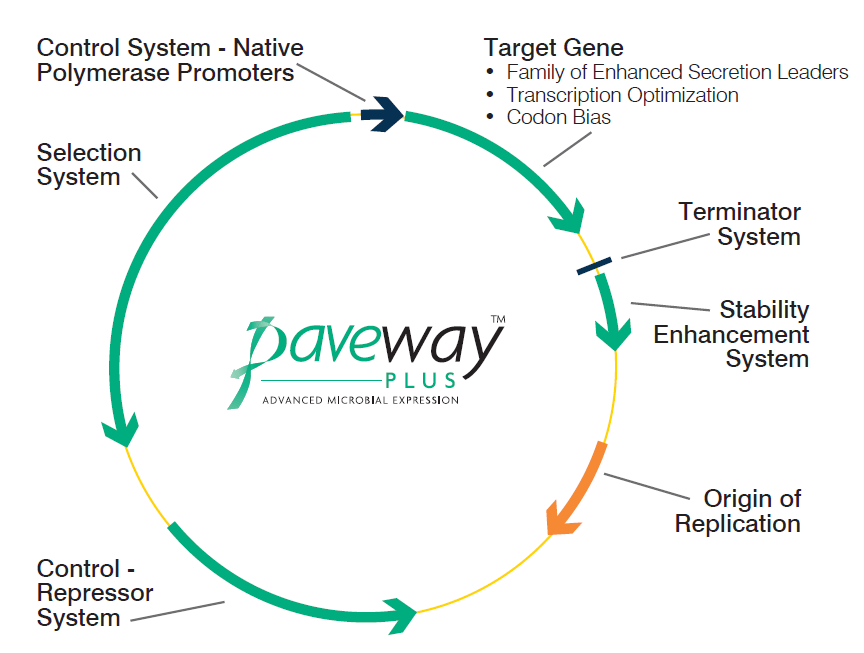
Figure 1.The family of Paveway plasmids for the high-titer expression and cGMP production of therapeutic proteins.
Tight control of expression of the cloned protein is made possible by the use of two perfectly palindromic lac operator sequences that are optimally spaced to provide a DNA loop in which the repressor tetramer can bind very tightly to the operators and completely shut off expression (Figure 2).

Figure 2. How the control system works: 1) plasmid DNA with free repressor tetramer; 2) repressor tetramer binds to a free operator; 3) repressor tetramer bound at first operator binds to second operator creating DNA loop which isolates the promoter from the gene of interest; 4) addition of IPTG dissociates the repressor tetramer, unravelling the loop and allowing transcription & translation to take place.
This ability to totally turn off protein expression until the cells are induced with IPTG allows high biomass accumulation prior to induction and assures that all cells are capable of protein production upon induction (Figure 3).

Figure 3. Tight control of protein expression in the Paveway system, showing cloned protein expression before and after addition of the IPTG inducer. No expression is detected before the addition of IPTG, and strong expression is observed after IPTG induction.
Such tight control also assures that the protein is not expressed prior to induction, avoiding plasmid instability due to metabolic load. Tight control of expression also helps to maximize titers, particularly of proteins that are potentially toxic to E. coli, and it enables a generic high cell density fermentation protocol to be used for any protein, shortening process development.
The expression level can be modulated by varying the IPTG inducer concentration (Figure 4), enabling the use of Paveway in situations in which the maximum rate of protein expression may not be needed for optimal accumulation of the appropriate form of the protein. For example, it may be beneficial to express a recombinant protein targeted for secretion or soluble intracellular expression slowly, so that the host secretion machinery or folding capacity is not overloaded. An overload can greatly reduce the growth and productivity of recombinant cells. Different combinations of the promoter region components can enable creation of a range of Paveway vectors with expression kinetics that can be tailored to the requirements of a specific protein and its production route. High titers of a diverse range of biopharmaceuticals can thus be generated using Paveway and the appropriate combination of host strain and fermentation conditions.
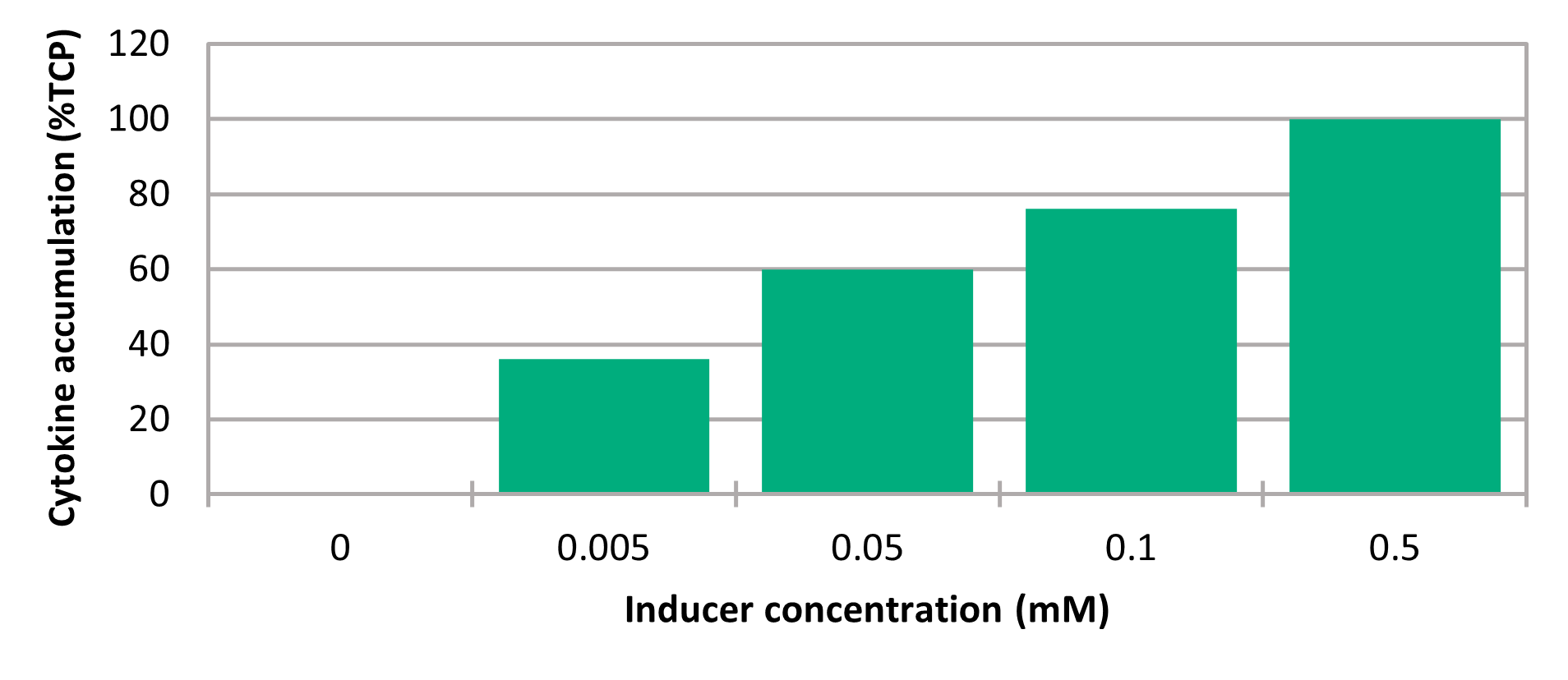
Figure 4. Modulation of protein expression levels by varying the concentration of inducer, using the Paveway system.
A panel of E. coli hosts has been developed that enables high expression levels and robust fermentation performance at high cell densities, as well as a set of platform fermentation processes suitable for laboratory and manufacturing applications. Paveway thus enables rapid selection of an optimal expression system and early definition of the upstream production process.
A Proven System
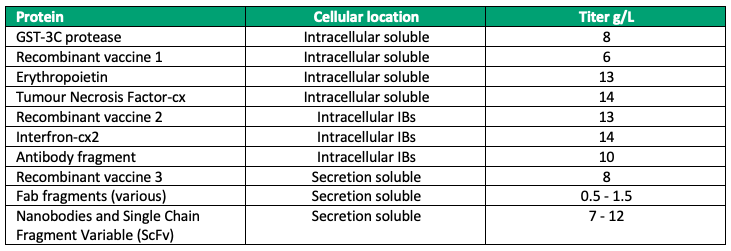
The unique features of this expression system have enabled the production of more than 140 different therapeutic proteins at very high titers since its inception over decade ago, from cytokines, fusion proteins, enzymes and vaccines to antibodies, ranging in size from 5 to 400 kilodaltons (Table 1). This confirms the ability of the system to generate highly productive recombinant E. coli strains with excellent scalability for biopharmaceutical production.
Table 1. Illustration of the wide variety of proteins that have been expressed by multiple routes (soluble, insoluble inclusion bodies, and secreted).
Fast Track Process Definition
We have a track record for enabling rapid entry into first clinical manufacture from recombinant plasmid construction. Starting from the protein of interest, we will explore the preferred route of expression and design rapidly to assess the best expression route. We will then clone the gene in a pre-defined set of plasmids, transform these into a variety of host strains and grow them in a range of conditions. This allows rapid selection of an optimal expression system and early definition of the upstream production process. The entire development process, from cloning of the gene of interest to fermenter evaluation, can be completed in just 6 weeks including assessment of titer. For clients selecting the Paveway PLUS advanced workflow, informed risk-based decisions on strain selection using product quality data can be made at week 11 (Figure 5).
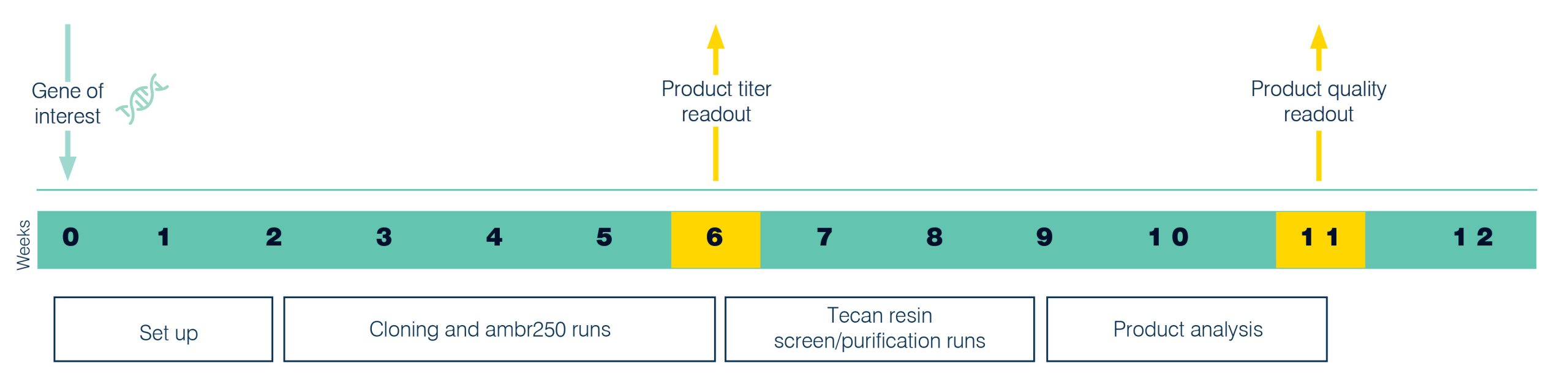
Figure 5. Fast track development of the cloned strain, the fermentation process and titer evaluation is completed in 6 weeks, while with the advanced Paveway PLUS workflow product quality data is available at week 11.
Using the ambr™250 system, both high-throughput strain screening and fermentation process optimization can be accomplished, thus speeding up process definition. Multiple design of experiment (DoE) fermentations can be carried out simultaneously in a scalable, reproducible system. This ensures that the maximum amount of statistically relevant data is collected using the minimum number of weeks. High quality data and process understanding can thus be obtained much earlier in the development process, and rapid progression is achieved from gene to high titer, strain specific, and optimized fermentation processes which are robust, well characterized and suitable for cGMP manufacture.
The downstream data analysis from the ambr™250 screening is also automated, further shortening the time to results. The Perkin Elmer LabChip® GX II automated electrophoresis system provides rapid and high-throughput analysis of expressed protein titers. We have developed custom scripts for effective mining of process data using JMP™ and can apply multivariate analysis to further define the design space at an earlier stage in the development timeline to get the most out of our data.
Success Stories
Our Paveway system has been used to successfully manufacture repeated batches of Theriva Biologics’ SYN-004, a proprietary oral beta-lactamase enzyme designed to degrade certain IV beta-lactam antibiotics excreted into the GI tract, thereby preventing antibiotic-mediated damage to the gut microbiome and reducing adverse outcomes associated with antibiotic-mediated gut dysbiosis. Initial manufacturing runs using the Paveway platform found that SYN-004 expression titers were increased by more than 25-fold compared to the Bacillus expression system used previously by Theriva Biologics. The SYN-004 manufactured using the Paveway platform has shown remarkable long-term stability and has been evaluated in a series of successful Phase 1 and Phase 2 clinical trials. These include a multinational, randomized, placebo-controlled study in 412 IV ceftriaxone-treated patients, where SYN-004 was found to protect gut microbiome diversity, lower the incidence of Clostridioides difficile infection (CDI) and reduce new colonization by opportunistic and potentially pathogenic microorganisms such as vancomycin resistant enterococci (VRE). SYN-004 is currently undergoing clinical evaluation as a means of reducing potentially fatal adverse outcomes associated with IV beta-lactam-mediated dysbiosis in allogeneic hematopoietic cell transplant recipients, including CDI, VRE colonization and bacteremia, and acute graft vs host disease (aGVHD). Theriva Biologics has been a long-term client of Fujifilm, citing the high efficiency and quality of the Paveway platform and Fujifilm’s sustained high level of customer service over the last 9 years.
Conclusion
The efficient expression of therapeutic proteins in microbial or mammalian systems is one of the major bottlenecks in the production of biopharmaceuticals. The combination of our unique and proven plasmids, fast process development and automated downstream analysis ensures rapid progression from gene to optimized, high-titer fermentation process. Our system also provides the in-depth process understanding and robustness required for cGMP manufacture and ability to efficiently express have been proven in more than 140 proteins of therapeutic interest. Furthermore, our Paveway PLUS advanced workflow launched in 2022 enables strain selection based on both product titer and quality, with lead strain identification within 11 weeks of program initiation.