Partnering for Success in Oncolytic Virus Development
By Michael Baker, Senior Director, Viral Gene Therapy, FUJIFILM Diosynth Biotechnologies
Oncolytic viruses (OVs) are an innovative and promising form of cancer therapy that leverages naturally occurring or intentionally engineered viruses to target and destroy a patient’s specific cancer cells while also stimulating innate and adaptive immune responses against these cells. This targeted and personalized approach has the potential to revolutionize cancer treatment by offering a more precise and less toxic alternative to traditional therapies like chemotherapy and radiation. In a previous blog post, we summarized the principal virus types, the main cell lines in which the viruses are produced, and briefly outlined critical aspects of OV manufacturing.
There has been a steady rise in interest surrounding OVs since the first approval by the US Food and Drug Administration of talimogene laherparepvec (T-VEC) in 2015. Numerous recombinant and non-recombinant viral oncolytic therapies are currently in clinical trial evaluation with adenovirus, pox, and herpes viruses emerging as the top candidates for development (Figure 1). The relatively short history of commercial success means no OV production process has reached “platform manufacturing technology” status on par with Chinese Hamster Ovary production systems for monoclonal antibodies.
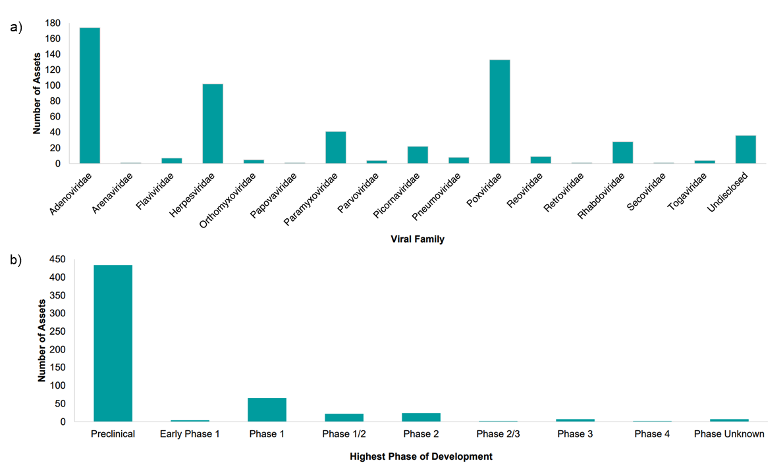
Figure 1. According to Beacon Intelligence H1 Oncolytic Virus Landscape Review 2023 published July 20231, a) the Adenoviridae, Poxviridae, and Herpesviridae families remain the most popular choices for developing oncolytic virotherapies with b) most assets in pre-clinical and early clinical phase (1, 1/2, or 2).
Given the wide variety of viral platforms, OV developers must navigate a gauntlet of decisions at each step of production that have implications for the success of their program. Partnering with a CDMO like FUJIFILM Diosynth Biotechnologies (FDB) with expertise in virus production, cell culture, and validation-level analytics can be hugely advantageous to OV developers looking to bring their products to market. FDB has the subject matter experts, specialized equipment, infrastructure, and established manufacturing and analytical platforms to help navigate decision-making at critical steps and provide solutions to key challenges in process development and scale-up to expedite OV programs.
Ensuring Patient Safety
Minimizing the potential for viral replication in healthy cells is critical to facilitating an OV candidate’s progression through the developmental pipeline. Cell lines including HEK293, HeLa and A548 can be used to produce replication-deficient oncolytic adenovirus. These cell lines provide the necessary early (E) genes that are deleted from the replication-competent adenoviral vector, which allows for viral propagation while minimizing the potential of generating replication-competent viruses. Nevertheless, there remains a concern about recombination events that could inadvertently generate fully replication-competent viruses placing an emphasis on factors during early phase development like transgene and vector design (i.e., promoters, regulatory elements). Transgene and vector engineering for OV is an active area of development to maximize their therapeutic potential against cancer while prioritizing patient safety by minimizing off-target events.
Adherent or Suspension Culture?
An important early decision on the path to Investigational New Drug (IND) approval is choosing between adherent and suspension cell culture platforms. Moving from pre-clinical to clinical stages and beyond, the implications will become increasingly apparent as the project scales. Vero cells, which are commonly used for OV development and manufacturing, rely primarily on adherent culture methods. However, adherent cell lines pose challenges for easy scale-up due to their requirement for increased surface area as production scales, labor-intensive handling, and higher contamination risk. Transitioning to suspension cell lines could be advantageous, as they are better suited for large-scale production and offer enhanced scalability compared to adherent cell lines.
FDB has established expertise in the development of both cell-based (suspension and adherent) processes. We have worked with clients to successfully adapt adherent cell lines to different culture formats for process intensification to increase cell yield, improve scalability, and enhance OV production:
- Adaptation of adherent cells to suspension culture in stirred tank bioreactors
- Microcarrier-based suspension culture in stirred tank bioreactors
- Transferring adherent protocols in cell stacks to more scalable technologies like fixed bed bioreactors
Also, the choice of cell culture medium affects the overall productivity, making media selection an important component for ensuring optimal scale-up.
Navigating Downstream Processing Hurdles
The envelope and budding process of viruses like herpes, pox, polio, can make them “sticky” and more difficult to release from host cells during production. Various methods are utilized for viral release including physical disruption or the use of salts such as dextran sulfate. It’s important to acknowledge that certain viral release methods are patented, which can pose challenges for OV development. To overcome potential IP obstacles, our team at FDB has prioritized the development of robust, non-patented alternatives for lysis. We’ve focused on developing and optimizing closed, single-use methods that are well-suited for large-scale production to bypass patent issues that could impede the advancement of developmental initiatives.
Like other biologic drugs, OVs need to be sterile when delivered for patient use to ensure the safety and efficacy. For adenovirus and other small, often non-enveloped viruses used in OV development, the final drug product can undergo terminal aseptic sterilization. However, larger viruses, such as herpes and pox viruses (dimensional axes of 0.242 and 0.303 microns, respectively) which cannot undergo the same sterile filtration steps, require aseptic processing from beginning to end. FDB has worked internally to develop an aseptic manufacturing platform process, which utilizes single-use, disposable assemblies that is readily deployable for clients working with these virus species.
Additionally, FDB has analytical and analysis capabilities designed for virus-based products for in-process evaluation and final product release analysis that are consistent with current regulatory guidelines.
CDMO Partnerships for Success
Overall, relying on the expertise of a CDMO with a proven track record in virotherapy is invaluable to advancing OV therapies to market. FDB has played a pivotal role in producing clinical grade materials for a leading OV candidate, which is presently undergoing advanced evaluation in late-stage clinical trials and demonstrating encouraging preliminary outcomes. The wealth of experience, problem-solving capabilities and leading-edge technologies that a CDMO partner brings allows OV developers to navigate the complexities of this cutting-edge field from development to late-stage clinical testing towards commercialization with greater ease and confidence.
For more information on FDB’s OV services, please visit: https://fujifilmdiosynth.com/cell-and-gene-therapy-services/
References
- Beacon Intelligence. H1 Oncolytic Virus Landscape Review 2023. Published July 2023. Accessed August 18, 2023. https://beacon-intelligence.com/infographic/h1-oncolytic-viruses-landscape-review-2023/
- Laine RF, Albecka A, van de Linde S, Rees EJ, Crump CM, Kaminski CF. Structural analysis of herpes simplex virus by optical super-resolution imaging. Nat Commun. 2015;6:5980. Published 2015 Jan 22. doi:10.1038/ncomms6980
- Baxby D. Poxviruses. In: Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 69. Available from: https://www.ncbi.nlm.nih.gov/books/NBK8364/