The Perfect Hosts for Vaccine and Virus Expression: Insect Cell Expression Systems
By Laura Walls, PhD, Technical Project Leader and Sharyn Farnsworth, MSc, Director of Upstream Development
Source: FUJIFILM Diosynth Biotechnologies
Choosing the right host is key to creating scalable, robust and reliable commercial processes
Baculovirus expression vector systems (BEVS) provide an efficient, flexible and scalable tool for the production of biologics in insect cells. Insect cells combined with BEVS are capable of producing high concentrations of multi-protein subunit complexes which are difficult to express in other cell types [1, 2]. BEVS large cloning capacity, ease of manipulation and potential for simple glycosylation and post translational modifications renders them an excellent tool for the rapid, cost-effective production of Virus Like particles (VLPs), Protein Subunits, Therapeutic Cancer Vaccines, Adeno-associated Virus and Lenti- Virus. As a result of the speed and convenience of BEVS/insect cell culture (ICC), they are well suited to manufacturing vaccines against rapidly mutating viruses [2, 3].
For over 20 years FDB has developed and commercialized multiple insect cell culture projects at various stages of readiness. Our knowledge helps clients define a strategy to develop, scale-up and manufacture BEV systems minimizing the time necessary to refine the process, ensure readiness with a line of sight to manufacture and ultimately commercialization.
Virus like particles as vaccines of the future
The 21st century has already encountered a number of major epidemic and pandemic events [4, 5], resulting in loss of human life as well as severe social and economic disruption globally. Traditional vaccines make use of live attenuated viruses (LAV) or inactivated virus technologies that generate a strong and lasting immune response, however these vaccines may not be suitable for immunocompromised individuals. As a result, VLPs have emerged as auspicious alternatives to traditional attenuated or inactivated viral vaccine due to their inherent safety and high immunogenicity.
Baculovirus expression vector system (BEVS)
BEVS has become an established platform for the manufacture of a wide range of viral vaccines and gene therapy vectors [1]. BEVS is summarized in Figure 1, briefly, insect cells are co-transfected with replication-incompetent baculoviral genomic DNA and a plasmid containing the gene of interest flanked by functional copies of essential replication genes [6]. Homologous recombination in vivo yields the replicating baculovirus capable of high-level production of the desired product, eliminating the need for a selection step.
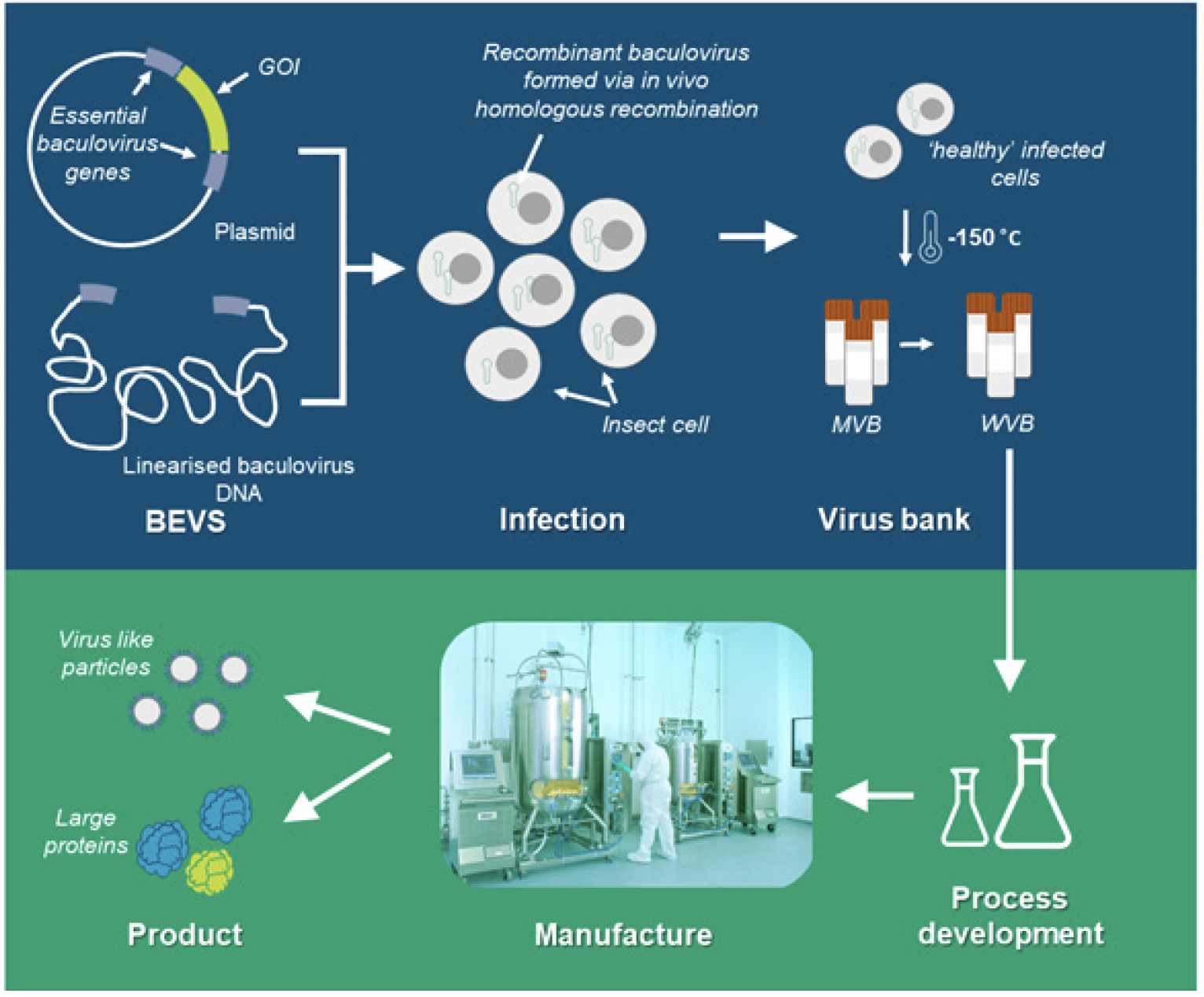
Figure 1: Example baculovirus expression vector system
Insect Cell Culture combined with BEVS has become the most widely used approach for the production of Very Large proteins and Virus Like Particles [3, 7]. The technology offers a number of key advantages over alternative expression platforms such as microbial or mammalian cell culture as summarized in Figure 2.
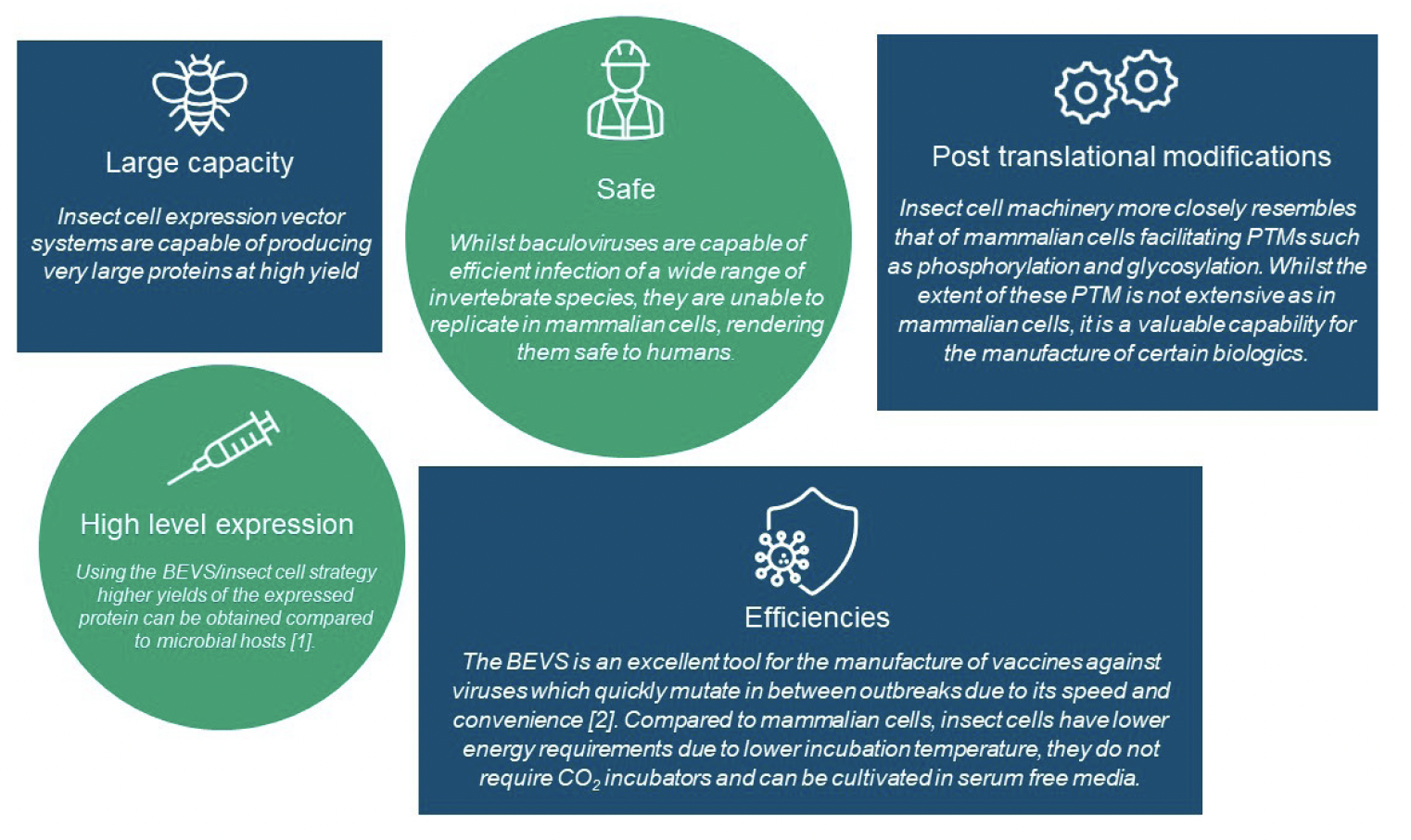
Figure 2: Advantages of the baculovirus expression vector system for Very Large Proteins and Virus Like Particles
Every step of the way, our BEVS Experts Deliver
The path from process development to commercialization is a well-travelled journey for our experts. Common challenges associated with the ICC/BEVS platform and FDB solutions are summarized in Table 1.
|
Consideration |
Typical Challenges Our Partners Face |
FDB Solutions and Approach |
|
Media |
• As nutritional requirements vary with process stage, media development that can go from seed train through infection can become a critical and variable part of establishing a long term strategy. • Use of complex media components adds variability to the process which may not be apparent in the short term of process development. |
• DOE-guided high-throughput media screening capabilities with the support of in-house statisticians. • Specialist teams generate and qualify scale down models for process parameter characterization. Close mimicry of manufacturing scale facilities results in effective process validation. • Establish a strategy to monitor the long term variability of undefined media components. |
|
Upstream and viral titer quantification |
• Need for robust viral titer quantification method. |
• Expert analytical development team on hand to develop robust quantification methods. • Specialist down-stream process development team with extensive experience in the purification of wide ranging protein products. |
|
Purification |
• Due to very large size of proteins, expression levels can be low (compared to typical mAb expression in CHO) and pose purification challenges.
• As the product is not a monoclonal antibody, standard protein A chromatography methods cannot be used. |
• Expert analytical development team on hand to develop robust quantification methods. • Specialist down-stream process development team with extensive experience in the purification of wide ranging protein products. |
|
Time restraints |
• With rapidly mutating infectious viruses, rapid vaccine development is essential to maximize efficacy. |
• FDB’s streamlined approach to analytical and process development with a line of site to manufacturing reduces risk and helps shorten timelines. |
|
Harvest |
• Productivity can be correlated with cell diameter and typically plummets once cell diameter and /or viability begins to decline from the peak post infection size.
• Proteolytic activity during the late stages of infection is detrimental to product quality and yield. |
• Capability for in-line measurement of cell size using state-of-the-art viability analyzers to reliably determine optimum harvest time.
• Use of flocculation techniques for improved filtration efficiency and deactivation of remaining live viruses. |
Table 1: Challenges in scale up and development of insect cell culture and BEVS
Summary
With over 20 years of experience in bringing baculovirus projects to commercial manufacturing scale at FUJIFILM Diosynth Biotechnologies, we have a tool-kit of solutions and expertise to ensure insect cell programs are successful at all stages of readiness from process development all the way to commercialization.
Contact us to discuss your science
Download the blogpost as a pdf
References
[1] R. S. Felberbaum, “The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors,” Biotechnology Journal , vol. 10, no. 5, pp. 702-714, 2015.
[2] C. M. Trombetta, S. Marchi and E. Montomoli, “The baculovirus expression vector system: a modern technology for the future of influenza vaccine manufacturing,” Expert Review of Vaccines, vol. 21, no. 9, pp. 1233-1242, 2022.
[3] S. Nooraei, H. Bahrulolum, Z. S. Hoseini, C. Katalani, A. Hajizade, A. J. Easton and G. Ahmadian, “Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers,” Journal of Nanobiotechnology, vol. 19, no. 59, 2021.
[4] K. Kusuma, P. Belagal, B. Viswanath and D. V. R. Sai Gopal, “Chapter 1 – Lessons learned from the first pandemic of the 21st century, global experience, recommendations, and future directions,” in Pandemic Outbreaks in the 21st Century, Academic Press, 2021, pp. 1-9.
[5] R. M. Meganck and R. S. Baric, “Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases,” Nature Medicine, vol. 27, pp. 401-410, 2021.
[6] O. Kolesnikova, A. Zachayus, S. Pichard, J. Osz, N. Rochel, P. Rossolillo, I. Kolb-Cheynel, N. Troffer-Charlier, E. Compe, O. Bensaude, I. Berger and A. Poterszman , “HR-Bac, a toolbox based on homologous recombination for expression, screening and production of multiprotein complexes using the baculovirus expression system,” Scientific Reports, vol. 12, no. 2030, pp. 1-11, 2022.
[7] S. Dai, H. Wang and F. Deng, “Advances and challenges in enveloped virus-like particle (VLP)-based vaccines,” Journal of Immunological Sciences, pp. 36-41, 2018.
[8] “Role of Recombinant DNA Technology to Improve Life,” International Journal of Genomics, vol. 2016, no. 2405954, p. 14, 2016.
[9] S. S. Paul, H. Trabelsi, Y. Yaseen, U. Basu, H. A. Altaii and D. Dhali, “Chapter 2 – Advances in long DNA synthesis,” in Microbial Cell Factories Engineering for Production of Biomolecules, Academic Press, 2021, pp. 21-36.
[10] S. Teworte, K. Malcı, L. E. Walls, M. Halim and L. Rios-Solis, “Recent advances in fed-batch microscale bioreactor design,” Biotechnology Advances, vol. 55, no. 107888, 2022