Striking a Balance Between Speed and Quality to Deliver Next-Generation Therapeutics
By Dr. Fay Saunders, Director of Mammalian Cell Culture, Process Development
Source: FUJIFILM Diosynth Biotechnologies
The therapeutic potential of bispecific antibodies (BsAbs) has become increasingly compelling for drug developers looking to generate molecules that unlock novel targets. BsAbs were originally engineered by chemically coupling two monoclonal antibody (mAb) fragments.1 Since then, their diagnostic and therapeutic usage has rapidly expanded thanks to developing technologies that produce them in a variety of sizes and structures.1 Currently, there are over 200 different types of bispecific molecules with more than a hundred under clinical evaluation for cancer treatment; most remain in the early development stages while four have been approved by the FDA.1 As more versions emerge, pharma companies and contract development and manufacturing organizations (CDMOs) must determine how to produce these molecules efficiently while maintaining control over quality attributes and safety profiles.
Bispecific molecules are more intricate than standard mAbs due to their ability to target two separate antigens. To achieve this bispecificity, they can have a higher number of chains that need to be expressed, typically three to four. One of the main challenges with bispecific antibodies is the mispairing of heavy and light chains that can occur during the expression of the molecule which allows the formation of unwanted product-related impurities, such as homodimers or undesired fragments (Figure 1). Molecule design can help minimize these, but their presence will require versatile strategies to provide material with high levels of purity.
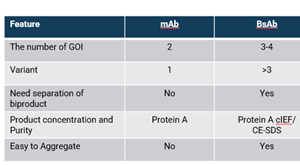
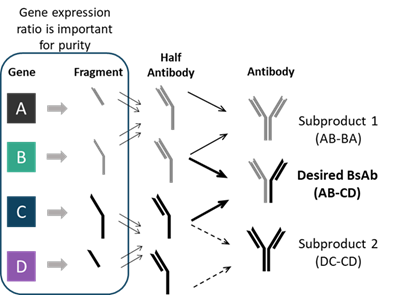
Figure 1. Gene ratio affects heterodimer/homodimer ratio
Meet Quality and Timeline Goals
Delivering a quality product quickly is critical and difficult to achieve without expertise. This is especially true for bispecific and multi-specific antibodies. Speed to clinic requires fast development, reliable material supply, and a robust, scalable process. This must be supported by product quality assessments that use advanced analytical techniques to monitor purity and cell line stability. When searching for a CDMO to manufacture your bispecific therapeutic, be skeptical of those that advertise the fastest possible timeline while potentially sacrificing your product specification goals. Instead, seek a manufacturer that threads speed and quality deliberately.
If a client is determined to move quickly, a knowledgeable manufacturer will emphasize that it is possible to be efficient while taking the time up front to ensure quality. At FUJIFILM Diosynth Biotechnologies (FDB), we can perform manufacturability assessments to help clients choose the most suitable candidate for cell line development (CLD) and de-risk the development process. Furthermore, we integrate enhanced product quality and characterization assessments into CLD to verify that the molecule is being expressed correctly with low levels of impurities. We also conduct product quality assessments during process development to certify reproducibility. These protocols provide our team with an early understanding of the timeline we can deliver material on, helping to establish efficiency, quality, and full transparency for our clients. On occasion, when a BsAb behaves similarly to a mAb, minimal optimization of FDB’s platform purification approach provides a viable means for rapid development and scale-up encompassing product quality assessments.
Explore the Four Pillars of Apollo™ X
FDB’s Apollo™ X is a robust, versatile, and scalable mammalian expression system with the ability to produce bispecifics and other high-complexity molecules. When approaching a bispecific molecule, we consider the necessary format for optimal expression. From there, we design the vector based on what ratios of genes need to be expressed. Typically, we conduct different transfections to determine optimal gene ratios and generate transfectant pools to look at expression and purity levels. Thus, the system is more diverse than one molecule, one vector, and one transfection.
Our Apollo™ X system provides a foundation for efficient, high productivity expression. The four key features of this system are:
- Expression vector: Apollo™ X uses random integration and an optimized dihydrofolate reductase (DHFR)-based selection system. This allows for stable characteristics of the cell lines and high expression levels without the need for amplification. FDB also has a proprietary leader sequence used for expression of all the molecules via Apollo™ X.
- CLD: Regulatory authorities need to see monoclonality of the cell lines in development. To ensure this, FDB uses the Cyto-Mine®, an integrated microfluidic platform which encapsulates single cells into picodroplets. Before depositing single cells into 96 well plates, we procure images of the single cells within the droplets. Post deposition, we image plates on the Cell Metric®, a high-resolution imager that provides visual evidence of monoclonality. We also employ predictive screens throughout the process. If molecules are compatible, we screen for productivity during the Cyto-Mine® stage and then use Ambr®15 to perform a final fed-batch screen at the end of CLD. Cell lines are placed into the upstream platform process that is representative of cGMP manufacturing so that we can identify cell lines to fit into our biomanufacturing process early, therefore mitigating the risk of needing to optimize process during subsequent scale-up. Our CLD process uses animal component free media and reagents throughout.
- Host cell line: During the development of Apollo™ X, FDB identified a superior CHO host cell line phenotype using adapted evolution, which entailed selecting a population out of the heterogeneous host that had improved growth and expression characteristics.
- Media feeds and bioreactor process: We use a proprietary, chemically defined medium during CLD and throughout process development. The screen conducted within CLD is the same process that runs at larger scales, making it scalable to 2,000 L and beyond.
Trust a Data-Backed Platform
In a study to assess bispecific expression in Apollo™ X, FDB tested how well each of the four pillars contributed to the expression of three bispecific molecules which had either 3 or 4 genes to express. Figure 2 demonstrates the molecules tested and the analytical approach required for purity determination; in this case, purity refers to the correctly formed heterodimer. Purity was assessed using differences in isoelectric points (pI) or molecular weights (MW) between homodimers and heterodimers. To determine the difference in charge, capillary isoelectric focusing (cIEF) was used, and to determine difference in MW, capillary electrophoresis sodium dodecyl sulfate (CE-SDS) was used.
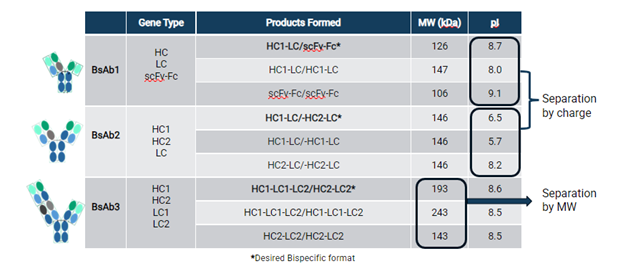
Vectors were constructed for the three molecules and transfectant pools were generated. Following recovery, fed-batch cultures were set up to assess growth, productivity, and levels of heterodimer. The pools with the highest product concentration and target purity were used to generate monoclonal cell lines. Following expansion into suspension culture, the cell lines were assessed in the Ambr® 15 fed-batch screen. For both BsAb1 and BsAb2, we were able to identify cell lines expressing the bispecific molecule at similar levels to what we typically observe for mAbs (Figure 3). The results were as follows:
- BsAb1: 70-94% purity, 3.73 g/L heterodimer
- BsAb2: 77-87% purity, 4.72 g/L heterodimer
- BsAb3: <80% purity, 0.26 g/L heterodimer
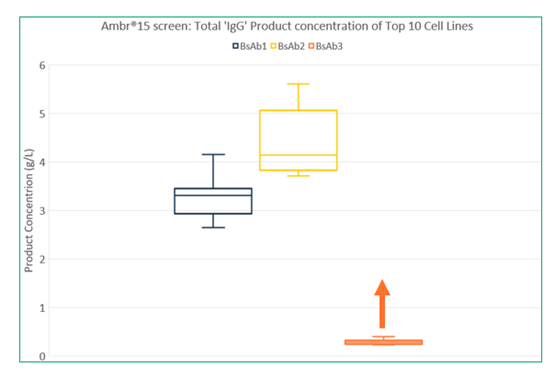
With BsAb3, FDB had to determine how best to troubleshoot the molecule to achieve higher expression. First, transcription efficiency was analyzed at the mRNA level which revealed limited expression of one of the chains. Further transfections were performed using varying gene ratios which increased the level of that particular chain, and higher expressing transfectant pools were generated. Our study results exemplify the importance of conducting early experiments to test these conditions.
To ensure efficient bispecific production, sponsor and CDMO must invest time up front to determine the optimal transfection procedure and the needed quantities of different vectors for high expression levels and desired product quality attributes. Following these early assessments, the process would follow a similar timeline to mAb production.
Invest in Experience
As a bispecific drug sponsor, you need confidence and security from your chosen partner. Take time during your exploratory phase to assess different CDMOs’ capabilities and expertise. Prioritize working with a partner that will achieve both speed and quality and has previous experience manufacturing BsAbs. This will help expedite delivery of a high-quality bispecific therapeutic to patients when they need it.
Much like bispecifics, Fc-fusion proteins have also become intriguing therapeutic modalities, consisting of a protein fused with the Fc region of an antibody and offering beneficial properties such as increased plasma half-life and stability. To learn more about how FDB is designing strategies to efficiently manufacture high-quality Fc-fusion proteins and bispecifics, check out this webinar. To explore Fc-fusion CLD strategies, read our whitepaper on the topic.
References
- Wei, J., Yang, Y., Wang, G., & Liu, M. (2022). Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Frontiers in immunology, 13, 1035276. https://doi.org/10.3389/fimmu.2022.1035276
About the Author

Fay Saunders is Director of Mammalian Cell Culture Process Development in the UK, having overall responsibility for CLD and process development activities. Fay has over 20 years’ industrial experience and has led the development of FDB’s Apollo™ X CHO expression platform. She has a Ph.D in Biotechnology from the Open University, investigating the role of chromatin modifying elements on mAbs production in CHO cells.
About FDB
FDB is a global CDMO operating in Europe and North America with integrated platforms to meet client demands and deliver medicines to patients faster. FDB has experience not only with drug substance manufacturing but also with finished goods manufacturing on both sides of the Atlantic, allowing a customer to work with a single provider for their end-to-end manufacturing needs. To learn more about their CDMO offerings, contact the FDB team.