Streamline Microbial Process Development to Reach Toxicology Trials Faster
By Steve Loftus, Microbial Business Steering Group Lead
Source: FUJIFILM Diosynth Biotechnologies
Microbial-based fermentation can yield major benefits for patients and manufacturers in terms of accessibility and cost-effectiveness. It allows for both faster processes and notable cost-savings; that said, it comes with inherent challenges and requires expert understanding and handling.1 Oftentimes, microbial-based products are unique, complex molecules that require specific processes that may not be platform-based. They require specific purification techniques and specialists that can think critically to adapt approaches.
In many cases, complex microbial-based biologics are born out of university offshoots and small biotech companies funded by private equity or venture capital firms who stage-gate funding. In such cases, reaching toxicology trials is a major step toward unlocking additional resources. The key is working with a contract development and manufacturing organization (CDMO) partner that has the knowledge and experience to navigate the inherent challenges of microbial fermentation and complex process development effectively and efficiently. For developers looking to quickly reach the toxicology (tox) trial stage with their microbial-based therapeutic, working with an experienced CDMO means that the program can be exclusively focused and intentionally tailored to develop a process for tox material generation and beyond.
The Challenges of Microbial Process Development
Despite its advantages, microbial process development (PD) often has to be customized for each molecule, which can make implementation of a standard platform process unrealistic; as a result, development must be done predominantly from scratch. Ideally, a manufacturer will be able to produce a high titer lead strain during strain development. Once it’s time to develop downstream processes, it is well-advised to build a new workflow from the ground up. Even with previous screening experience, manufacturers often encounter new obstacles. A large part of microbial PD is spent screening new chromatography resins and tangential flow membranes coupled with optimizing their operating conditions.
Purification processes vary widely from molecule to molecule. If a molecule is expressed as inclusion bodies, it typically needs to be refolded, with refolding conditions specific to the protein of interest. You may need to navigate misfolded forms and charge variance. For soluble molecules, you’ll face similar challenges around the separation of different forms as well as potentially more complex separation procedures where host cell proteins must be flocculated to enrich the clarified material going into the downstream process. To navigate these difficulties, it is critical to work with a manufacturer that takes an iterative approach to development by gathering data and making real time adjustments to the process while communicating developments and presenting options that have longer term viability.
The Gene-to-Tox Solution
At FUJIFILM Diosynth Biotechnologies (FDB), our PD teams have a deep and extensive knowledge of the development of microbial-based biologic processes. This range of manufacturing experiences has enabled our teams to design a streamlined development strategy to help sponsors quickly reach preclinical tox trials. Specifically, the Gene-to-Tox program is a set portfolio of services structured to efficiently progress microbial biologics to tox trials and includes the following:
- Strain development in E. coli or yeast (P. Pastoris) with top producing strains identified in as little as 6 weeks
- An 11 week expanded workflow (including strain development) to assess titer of top strains and early product quality (PQ) analysis, buffer screening and capture column definition
- Full PD with a minimum of two chromatography stages and one tangential flow filtration (TFF) step to bring product to sufficient purity as well as a suite of analytical testing methods to assess the product prior to release for tox trial
- Lab-scale run to generate material for further assay development or client use
- Pilot 100 L scale run to generate material for tox trial
- Liquid formulation, no higher than 10 mg/mL
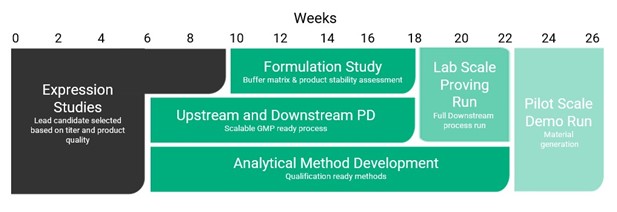
Overall, it’s a shorter, leaner, and more affordable program for developers looking to reach tox trials efficiently. While traditional PD aims to take clients all the way through to cGMP manufacture, our Gene-to-Tox workflow allows for a natural break in process development. At this stage, clients can conduct toxicology trials and progress to Phase I clinical manufacturing when ready. Leveraging this set package of work allows for an efficient process and a manageable scope of work for sponsors who may not have taken a molecule through to cGMP manufacture previously. FDB also offers the benefit of additional modular packages which can be added to address specific client needs. Thanks to our streamlined development approach, buffers, resins, and membranes are procured from trustworthy vendors to ensure they are well-stocked or easily accessible.
Though Gene-to-Tox is tailored towards pre-cGMP activities, once complete cGMP preparation can commence at an agreed time point. At that stage, it is effortless to resume thanks to our experience optimizing and scaling up processes to commercial scale. For large pharma partners looking to outsource their early phase development, FDB can offer an extended workbench to rapidly develop processes and provide material for a library of pipeline candidates. Developers can be secure in the knowledge that the processes will be developed with scalability in mind, reducing the amount of redevelopment required once a lead candidate has been identified to progress to clinical manufacture.
References
- Mirasol, F. (2022, October 1). Weighing the benefits of fermentation for new Biotherapies. BioPharm International. https://www.biopharminternational.com/view/weighing-the-benefits-of-fermentation-for-new-biotherapies
About FDB
FUJIFILM Diosynth Biotechnologies, a subsidiary of FUJIFILM Corporation, is a global CDMO partner for the development and manufacture of biologics, vaccines, cell and gene therapies, and oncolytic viruses. The company operates a global network with major locations in the U.S., the U.K., and Denmark. FDB has over thirty years of experience in the development and manufacture of recombinant proteins, vaccines, monoclonal antibodies, viral products, and medical countermeasures expressed in a wide array of microbial, mammalian, and host/virus systems. For more information, go to https://www.fujifilmdiosynth.com.