Navigating the Allogeneic Commercialization Journey: The Challenges and Triumphs
Introduction
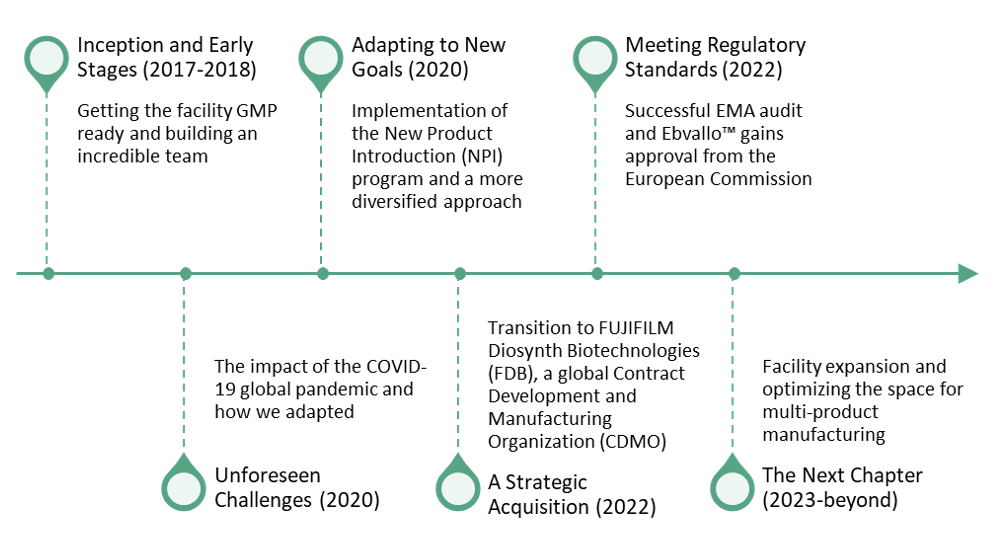
The journey towards preparing a GMP facility for an allogeneic cell therapy product’s commercialization began with the construction of our cell therapy facility in Thousand Oaks, CA. This endeavor was far from straightforward, marked by many twists, turns and challenges along the way, from the unforeseen global COVID-19 pandemic, to introducing pre-IND programs, to the acquisition by FUJIFILM Diosynth Biotechnologies (FDB) to become a CDMO.
At every step of this journey, our dedicated team contributed fresh ideas and innovative solutions. Each challenge we faced became an opportunity for growth, and each success fueled our unwavering commitment to advance our customers’ programs for the benefit of patients.
In this article, we will walk through the journey and highlight the pivotal moments, challenges, triumphs and lessons learned that have shaped our evolution from facility construction to a fully operational, patient focused CDMO.
Inception and Early Stages (2017-2018)
The construction of the 90,000-square-foot facility officially began in 2017 and opened in July 2018 with the inaugural GMP lot produced in December 2018.
From the very outset, our vision for this facility was focused on advancing a single allogeneic cell therapy product to commercialization. This approach influenced not only the facility’s design but also the personnel we recruited. We added highly skilled personnel across the QA, QC, Manufacturing (MFG), and Supply Chain teams to help enable commercial readiness.
One of the early lessons learned came from our experience with Manufacturing Execution System (MES) validation. As we moved forward and a robust MES was implemented and validated, we quickly learned that it did not provide enough flexibility for updates and early phase program modifications. Our team was able to navigate this challenge and adapt our processes effectively, which taught us the importance of building phase-appropriate flexibility in our systems and processes from the start.
Unforeseen Challenges (2020)
Amid the global upheaval caused by the COVID-19 pandemic, our facility, like many other companies across the globe, encountered a series of unprecedented challenges that put our adaptability and resilience to the test. California’s statewide shelter-in-place order on March 19, 2020, prompted 90% of our staff to transition to remote work. To ensure production continuity, we had to quickly pivot and adapt our workflow. We were able to maintain production and clinical shipment schedules without delays or batch losses thanks to a well-defined strategy with risk assessment and troubleshooting that prioritized employee safety and communication.
Remote work and contact tracing impacted our QC team especially since they had to support both internal and external testing. To address on-site cell culture needs, the QC team cross-trained MFG staff to help maintain lab productivity and this had the added benefit of fostering stronger bonds across the departments.
The pandemic also increased our reliance on electronic systems to manage remote work. We solved paper-based QC documentation backlogs through the implementation of a Laboratory Information Management System (LIMS) and we took proactive steps to ensure that all our processes were up-to-date on the digital platform. Although effective, information migration was performed without establishing metadata standards, which made it very difficult to find and retrieve information within the system. It really emphasized the importance of having a standardized metadata strategy in place to operate effectively within a digital environment.
Supply chain disruptions at the height of the pandemic led us to rethink and optimize our material management strategies. We implemented initiatives to source new suppliers for critical raw materials and created backup plans in the event of potential future supply disruptions or shortages.
In the face of these unprecedented adversities, we learned how to focus on what’s critical, how to adapt to change during challenging times, and importantly, the value of creating contingencies and redundancies both in our staff and in our supply chain. Embracing “just-in-time” systems and meeting chaos with determination, we emerged more resilient, agile, and better prepared for the next phase of the journey.
Adapting to New Goals (2020)
In October 2020, the New Product Introduction (NPI) program was initiated, which expanded the focus of our facility, beyond supporting a single commercial allogeneic program. A more diversified approach to encompass early-phase programs required us to reevaluate and adapt our documents and systems. During our first NPI, we gained valuable insights, quickly realizing we needed scalable and omni-product SOPs and systems. For example, products in early-phase development have more flexibility within the current Good Manufacturing Practices (cGMP) framework compared to a commercial program, so building in phase-appropriate flexibility would help us accommodate a wider scope of programs. The experience we gained during COVID-19, while at times painful, taught us important lessons about flexibility and adaptability that ultimately helped set us up for success when we were implementing the NPI program. These principles enabled the successful introduction of 6 programs ranging from pre-clinical to PPQ enabling.
A Strategic Acquisition (2022)
In April 2022, FUJIFILM Corporation acquired Atara Biotherapeutics, Inc.’s cell therapy manufacturing facility to be part of FUJIFILM Diosynth Biotechnologies (FDB), a Contract Development and Manufacturing Organization (CDMO). This transition marked the beginning of a new chapter, as we shifted from being a independent biotech innovator with a specific pipeline to taking on the role of a global CDMO. This brought with it some uncertainties and questions that we needed to address:
- How do we adapt to FDB standards?
- How do we adapt our existing processes and systems to support a diverse range of modalities, manufacturing scales, and compliance levels?
- How can we keep supporting the pipeline goals for our first client?
- How can we make sure we’re ready for the EMA audit to support registration in Europe?
To navigate some of these challenges, we launched an initiative to enhance the adaptability and phase-appropriateness of our systems, aiming for flexibility and agility while maintaining compliance. While we had initiated this process with NPIs, it was clear that we needed to apply it on a broader scale: we started this journey focused on 1 product, and then expanded our strategy to a whole pipeline. Now, we also needed to take into consideration the depth and breadth of the cell therapy industry. Instead of building in every scenario for every client program, we leveraged quality agreements to create phase-appropriate requirements. This enabled us to align more closely with our clients’ needs and utilize appropriate systems to ensure that our efforts yielded the necessary results for advancing their programs.
And from our experience with NPIs, we needed phase-appropriate materials solutions to serve clients with programs in various stages of development. To do this, we engaged in strategic partnerships with our suppliers, which involved hands-on visits to their facilities that ultimately led to positive improvements at our supplier’s facilities. We found our suppliers were willing to work closely with us to meet our requirements thanks to these partnerships, including implementing controls to reduce particulates on starting materials.
During our transition, we encountered occasional discrepancies between internal and external stakeholders regarding expectations for production runs, which posed some challenges related to resource allocation and data access. To address these issues, we actively worked on defining standardization levels in terminology, processes, and resource management. These efforts aimed to enhance our ability to manage expectations effectively as we assumed our new role as a CDMO. At times, we also faced challenges in harmonizing our language and experienced conflicts related to resource allocation and data access. Maintaining close relationships with our supply chain partners and customers enabled us to effectively address all these challenges and provide win-win solutions wherever possible.
Post-acquisition, the facility moved from using Microsoft D365 as its primary enterprise software solution to SAP HANA. Incorporating lessons from the D365 implementation, we built in collaboration from the start to avoid siloed implantation and ensure consistency and flexibility in our solutions.
The acquisition process happened within a relatively short time span, which required extensive coordination to maintain work schedules during the transition and we experienced system “blackout periods” that were challenging but necessary to migrate to FDB systems. FDB’s network-wide support played a pivotal role in the transition and in acclimating our entire facility to our new responsibilities as a CDMO such as enhancing our customer service skills, building client agnostic processes, and establishing connections to the larger FUJIFILM network to ensure our offering could effectively address a diversity of customer needs. By leveraging this global collaborative network of experience, we were able to ensure we were cGMP functional from day 1 and resulted in zero impact to product distribution or production.
Meeting Regulatory Standards (2022)
In April and May 2022, our facility underwent the much-anticipated audit by the European Medicines Agency (EMA), a significant milestone in our journey towards commercialization. An interesting fact is that due to COVID-19 restrictions, the audit was conducted remotely on German time, a testament to the adaptability required during the pandemic.
In our preparations for the EMA audit, we ensured that both our QC and MFG systems were aligned with EMA standards and regulations. On the personnel side, we conducted mock audits that provided valuable practice and confidence-building opportunities. These preparations were instrumental in our successful EMA audit, which we passed with flying colors. In December 2022, Ebvallo™ gained approval from the European Commission.
Lessons Learned and Key Developments
Throughout our journey, we’ve learned invaluable lessons that have shaped our approach and behaviors:
- Planning across the entire value chain and establishing redundancies where possible is important. We learned this the hard way when production-focused scheduling maxed out QC resources. This taught us to plan across the entire value stream to ensure that manpower and materials are allocated appropriately.
- Establishing a clear roadmap has proven essential for maintaining focus on our long-term goals. In the past, we experienced challenges because we lacked the necessary documentation to guide our systems, leading to organic and sometimes disjointed growth.
- We learned during COVID-19 that having a single source of truth with an enterprise software has significant benefits, but standardizing metadata is equally vital for data management and retrieval.
- Defining clear roles and responsibilities for personnel is not easy but essential when supporting new and challenging programs. Building the right structure within our departments to enable flexibility, enhance communication and leverage strengths helped us get through COVID-19 and has continued to serve us well.
- Despite the unique nature of each cell therapy product, we’ve discovered that some degree of standardization can be applied through our accumulated experience to reduce errors and enhance our systems. For instance, we implemented platform-style operator training, which streamlined Aseptic Operator Qualifications.
- We implemented an Aseptic Coaching Program that has improved staff skills, reduced particulate defects, and strengthened existing contamination controls for the benefit of all programs at the facility.
- We learned that building a strong backbone in QC methods based on core quality control elements before layering on additional complexity is key to ensuring the overall consistency and the reliability of our methods.
- During routine production runs, we found it was extremely beneficial to have MSAT (Manufacturing Science and Technology) and QA staff on the floor for real-time support and as first response during unplanned events or deviations from the standard process to triage issues, perform root cause analysis, and make informed decisions regarding process adjustments or corrective actions.
- And finally, as a CDMO, it is important to understand needs especially with programs in different stages from pre-IND to late phase. It’s crucial to know the immediate needs compared to those necessary for eventual commercialization. This is a work-in-progress, and our QA is still evolving and improving because regulatory standards in the cell and gene therapy space are continually changing as more products advance through development and reach the market.
Celebrating Our Achievements
Along the journey to commercialization, we encountered and overcame many obstacles that are worth celebrating. We genuinely believe in the importance of putting patients first. Even during the height of the COVID-19 pandemic, when remote work and contact tracing became the norm, our staff went above and beyond. They willingly put in extra hours to ensure that patient clinical shipments and cGMP lots remained on schedule.
Our “Lessons Learned” sessions have become an integral part of operations at our facility. These sessions occur after every campaign, NPI, or significant obstacle we’ve encountered, giving us time as a collective to critically analyze events. This has helped to drive improvements and establishing best practices for future projects.
This feeds into our strong company culture and team-oriented mindset—we struggle together, and we celebrate together. Having supportive leadership has helped to foster this environment of teamwork, with individuals eager to join and remain part of our team. During the acquisition, we successfully transitioned 140 individuals, and we consider ourselves fortunate that many of the operators who joined our facility in 2018 are still an integral part of our team today.
We are also immensely proud of our NPI program with the successful execution of seven NPIs in just four years across a wide range of products, including viral vectors, pre-IND CAR-T therapies, and commercial-ready cytotoxic T cells.
And, finally, there was the EMA audit—an incredible opportunity for growth and an invaluable learning experience for our facility. To see this journey culminate in the approval of the first-ever allogeneic T-cell immunotherapy is nothing short of amazing. And to many of us who had been on the commercial journey since the start, the approval wasn’t just a professional achievement, it was very much a personal one.
The Next Chapter (2023-beyond)
Our journey to commercialization was undeniably challenging, filled with obstacles and unexpected turns. Yet, these very challenges became opportunities for growth and learning. Every hurdle we faced served as a stepping stone that taught us important lessons to help us improve and propel us forward. The acquisition marked a pivotal moment in our evolution, with our facility and staff transforming from a modest startup into a mature, experienced CDMO partner, all while maintaining the nimble mindset of a startup, which allowed us to readily adapt to evolving and unexpected circumstances. Through it all, our core mission and values have remained the same: the patients matter, and we are committed to delivering life-saving treatments to patients in need.
But the journey doesn’t end here. As we look to the future, the road ahead holds new challenges and opportunities as we optimize our facility for multi-product manufacturing. Originally designed for a single allogeneic cell therapy product with many open processes, we are expanding the facility to accommodate closed processes and automation. This will allow us to create flexible spaces that are adaptable to various modalities and evolving regulatory standards in the ever-changing cell therapy landscape.
Now that the path to commercialization has been traversed, we can use the wellspring of knowledge we’ve gathered as well as our roots as a cell therapy innovator company and commitment to advancing lifesaving therapies to help other cell therapy companies reach the same level of success. We will continue to push the boundaries of innovation to enable broader patient access to life-saving cell therapies. Our resilience, adaptability, and determination forged through this journey will help us overcome any new challenges in the future and ensure that we remain committed to putting the patients first.
If you’d like to know more about how FUJIFILM Diosynth Biotechnologies can help you with the development and manufacture of your cell therapy project contact us directly here.