Leveraging Advanced Analytical Capabilities to Derisk Drug Development
By Jason Barker, Director of Analytical Development, Analytical Methods Transfer and Formulation Development
Source: FUJIFILM Diosynth Biotechnologies
As biotherapeutic molecules continue to grow increasingly complex, it is vital for sponsors to have a thorough understanding of structural properties and product- and process-related impurities to create thorough regulatory submissions. For example, the use of fusion proteins and bispecific molecules in therapeutics has necessitated a more diverse analytical toolbox, including mass spectrometry, biophysical characterization, automated sample preparation, and high throughput analytics. Beyond helping to ensure compliance, gaining an early understanding of a molecule’s characteristics informs strategy and decision-making throughout the product development cycle.
To guide complex biopharma products to the approval stage, sponsors and their manufacturing partners will need to enlist non-platform analytical methods. The team at FUJIFILM Diosynth Biotechnologies (FDB) works under the principle that every protein has its own personality, and said personalities drive the need for extended characterization. A protein’s personality is primarily defined by its structural properties but also potentially by product- and process-related impurities. Building thorough knowledge of protein identity and behavior using advanced analytical methods yields benefits that will extend across the product development lifecycle.
The Importance of Accommodating Molecular Diversity
A February 2021 report from the Biotechnology Innovation Organization found that the overall likelihood of a Phase 1 biopharma development candidate making it to regulatory agency approval was 8% from 2011 to 2020.1 Only 29% of molecules made it through to Phase 2 during this timeframe, and the average timeline from Phase 1 to commercial licensure was 10.5 years. Since FDB’s offerings encompass everything from preclinical development to commercial manufacturing, it is critical to maintain a suite of analytical characterization techniques to support partners.
To fully characterize molecular structure and impurity profile and enable efficient process development and successful manufacturing, FDB has developed a suite of extended characterization packages to support IND and BLA submission chapters 3.2.S.2.1 Elucidation of Structure and 3.2.S.3.2 Impurities. These packages are centered around techniques to elucidate both primary and higher order structure, understand product-related impurities, and characterize process-related impurities, such as host cell proteins (HCP).
Analytical Tools to Elucidate Molecular Structure
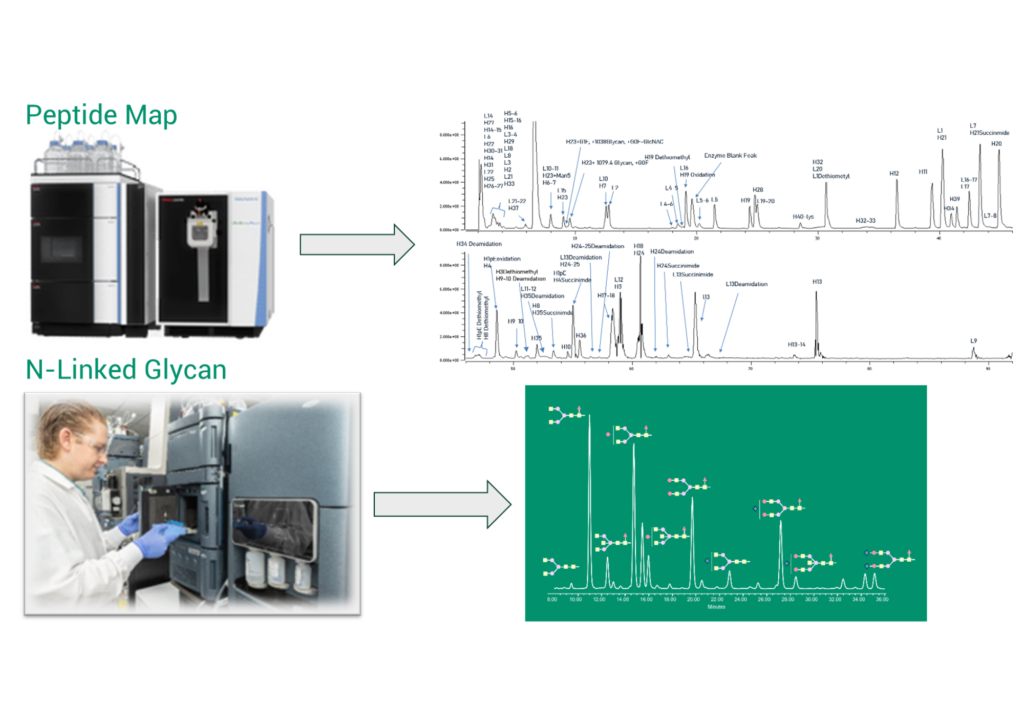
FDB’s approach to analytical characterization begins with peptide mapping using liquid chromatography and the Orbitrap mass spectrometer to confirm the amino acid sequence coverage and identify post-translational modifications, such as deamidation, succinimide formation, and sites of glycosylation (Figure 1). Figure 1 also shows a well-established workflow to identify glycans released by enzymatic treatment of a protein using hydrophilic interaction liquid chromatography (HILIC) and time-of-flight mass spectrometry (TOF-MS).
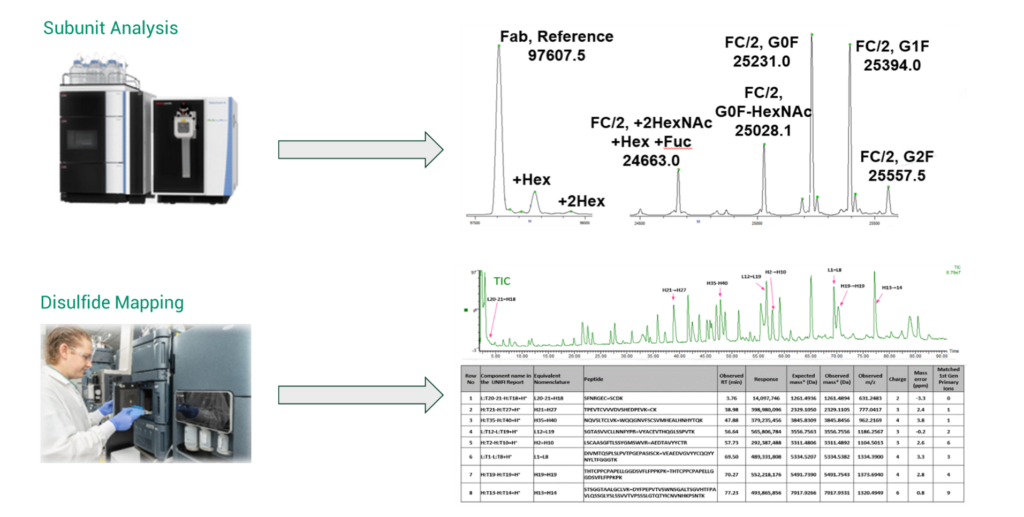
FDB also has a standard workflow for subunit analysis of monoclonal antibodies (mAbs). This technique utilizes IdeS, a highly specific cysteine protease (IgG-degrading enzyme of Streptococcus pyogenes), to break a mAb into Fc/2 and Fab fragments (Figure 2), which can also be used to determine the post-translational modifications of a mAb, such as glycosylation. Figure 2 also shows a disulfide mapping workflow that uses an enzymatic digestion with and without reducing the disulfide bonds present to map the cysteine residues where disulfide bonding occurs.
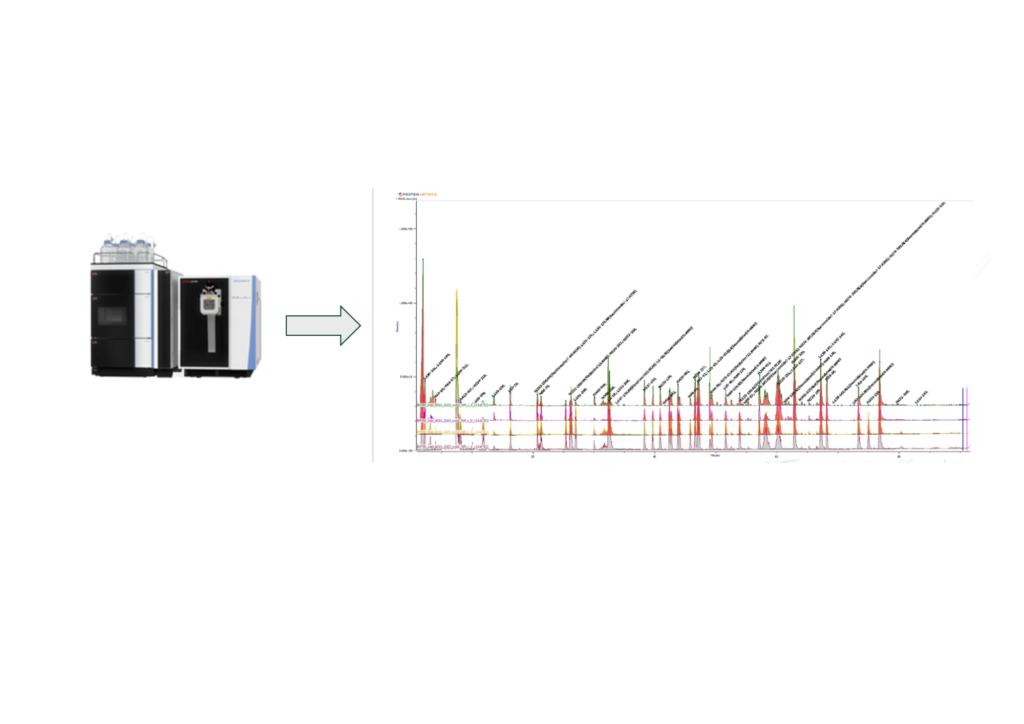
The final mass spectrometry example (Figure 3) for primary structure elucidation is sequence variant analysis or SVA. This workflow leverages the high resolution and sensitivity of an Orbitrap mass spectrometer to evaluate single amino acid variants due to DNA mutation or process misincorporations down to levels of about 0.1%. In Figure 3, sequence variant analysis workflow was used to identify a valine lysine misincorporation that resulted in an unidentified peak in a standard peptide mAb workflow.
Strategies for Biophysical Characterization
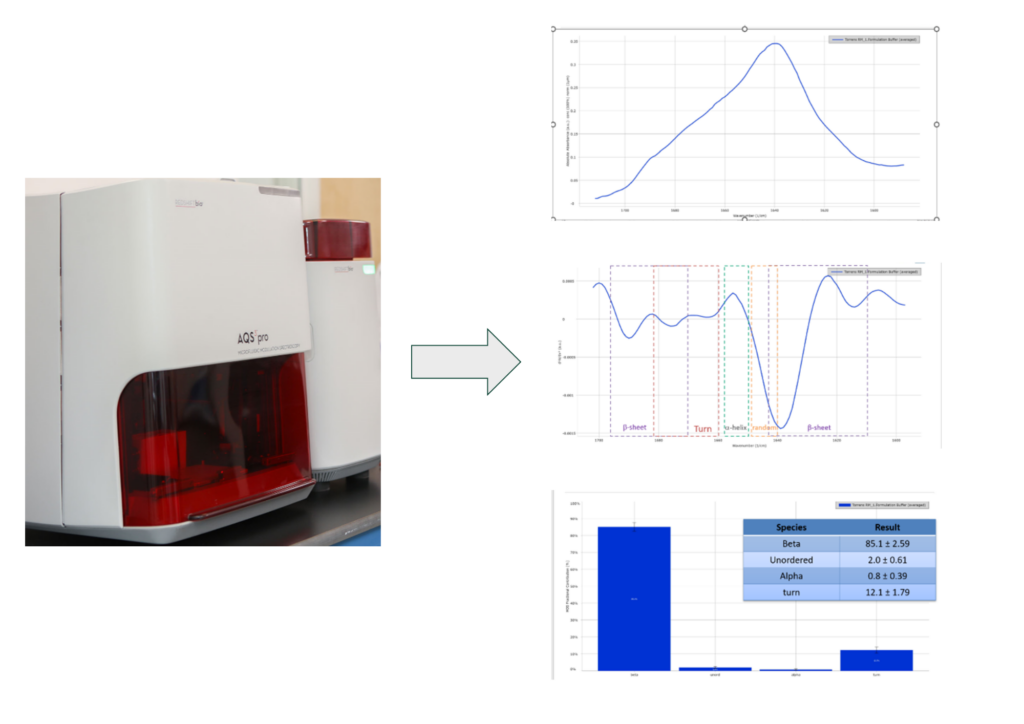
The FDB formulation development team has extensive biophysical characterization capabilities that interrogate the higher order structure and thermodynamic stability properties of a molecule prior to formulation development studies. Fourier-transform infrared (FTIR) microfluidic modulation spectroscopy utilizes a powerful laser to conduct FTIR on a sample and buffer reference in continuous flow via micro channels. This technique provides secondary structure information, alpha helix, beta sheet, beta turn, and unordered structures content, for samples at concentrations of up to 200 mg/mL without any dilution or preparation (Figure 4).
Size exclusion chromatography (SEC) is a standard technique for determining the presence of size variance under native conditions for biopharmaceutical products. The following scenarios demonstrate how SEC can sometimes provide misleading information. In Figure 5, SEC analysis of a mAb revealed a peak eluding prior to the main product peak. Based on elution order, the standard assumption would be that this peak represented a high molecular weight aggregate species. However, FDB utilized online multi-angle light scattering to determine that the molecular weight of this peak was consistent with the monomeric species, and therefore, it was not an aggregate, providing valuable information to our formulation and process development groups.
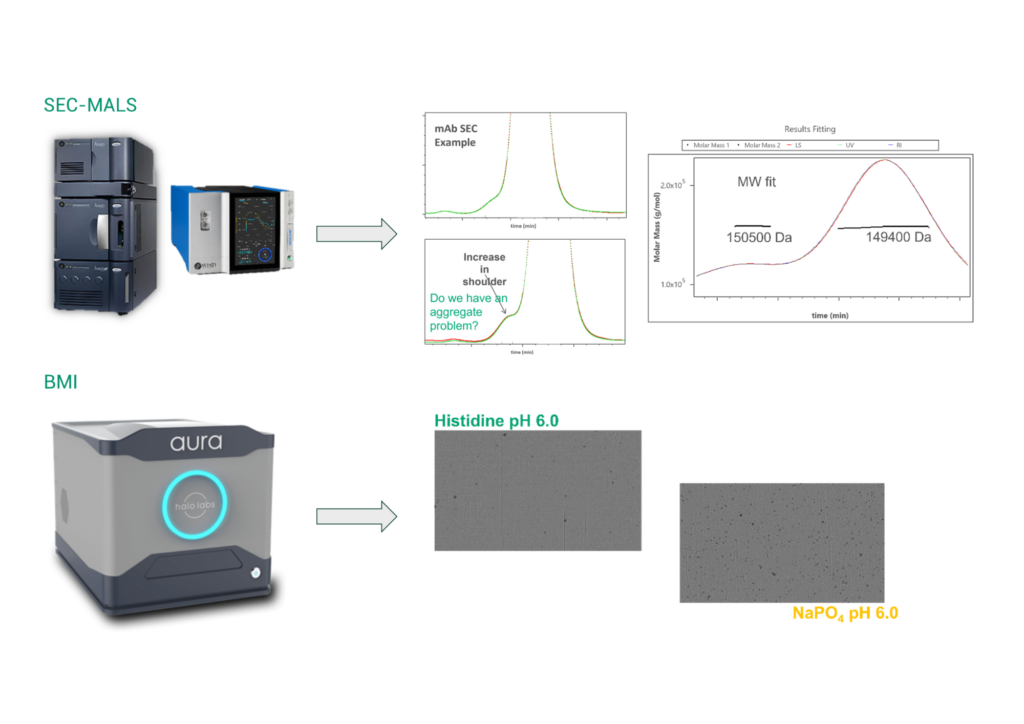
Backgrounded membrane imaging (BMI) utilizes a 96-well membrane plate to isolate subvisible particles which are subsequently imaged. Since the particles are imaged against air instead of a liquid background, the refractive index contrast is ten-fold greater than standard light obscuration detection, which yields highly sensitive detection of subvisible particles. Additionally, subvisible particles in the 0.1- to-100-micron range are often trapped on the front of an SEC column and not detected. Per Figure 5, SEC data for these two formulations looked equivalent; however, BMI revealed significantly higher subvisible particles content in the phosphate formulation.
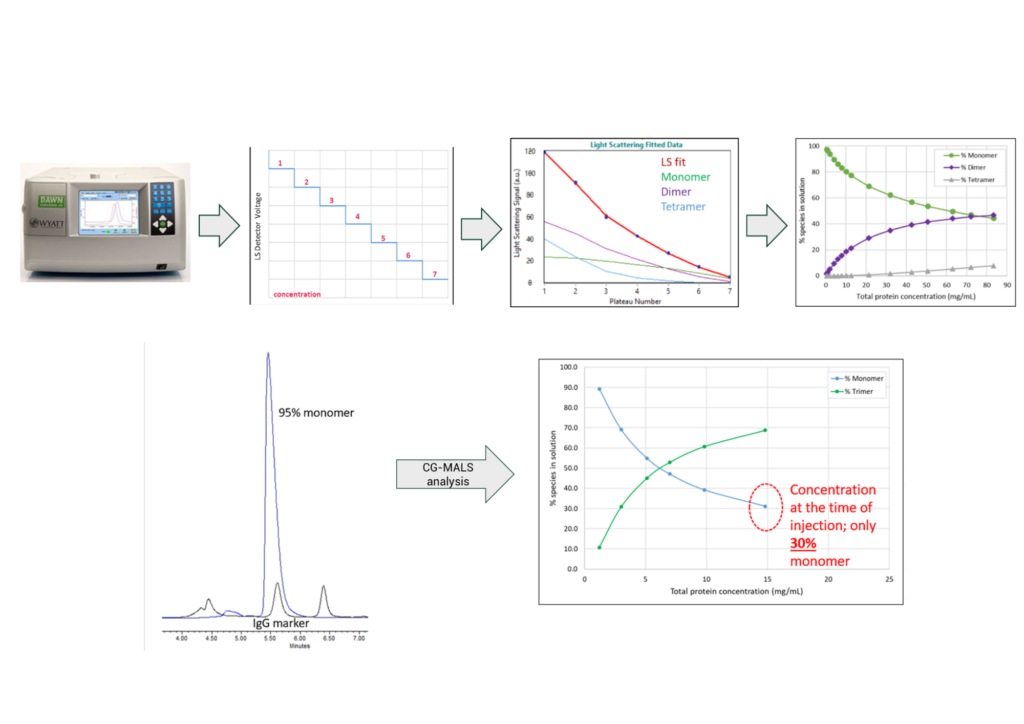
Concentration gradient multi-angle light scattering (CG-MALS) measures molecular weight at varying protein concentrations using multi-angle light scattering signal in batch mode rather than after an SEC separation. The change in molecular weight across a concentration range is then used to model reversible associations, revealing more about the solubility and colloidal stability of the molecule. A decreasing molecular weight alongside increasing concentration indicates repulsive interactions between molecules, while an increase in molecular weight with increasing concentration indicates attractive interactions. These interactions may not be accurately depicted by SEC due to the dilution that occurs when a sample is injected onto an SEC column. In Figure 6, SEC for this sample indicated 95% monomer. However, the CG-MALS method showed that prior to the on-column dilution, the molecule was only 30% monomer.
Techniques to Characterize Product-Related Purity
Historically, characterization of product-related impurities has required fraction collection. To characterize acidic or basic variance, manufacturers had to adapt a pH gradient ion-exchange chromatography method to maximize the load and fraction yield mass without impacting resolution. Following method optimization, 30 to 50 injections were conducted to obtain sufficient material for characterization. The next steps included buffer exchange, material concentration, and re-analysis in the parent method to confirm that representative fractions had been collected. Recovery through this process was often 50% or lower, and there were plenty of opportunities for degradation. Similar efforts were required for SEC.
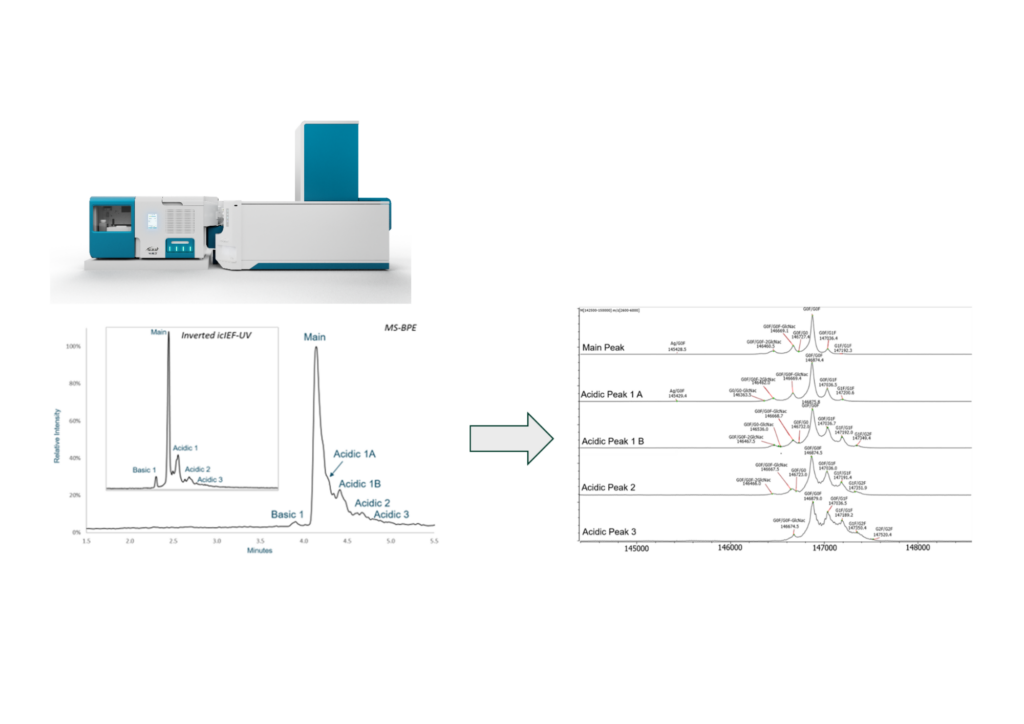
Over the last several years, FDB collaborated with SCIEX on a demonstration of the new Intabio imaged capillary isoelectric focusing mass spectrometry (icIEF-MS) system with the ZenoTOF 7600 mass spectrometer. This interface enables icIEF separation using carrier and flight compositions equivalent to the parent test method. The interface allows the separation to be directly infused into the mass spectrometer and has shown that the separation matches an icIEF profile generated with the Maurice tool and a standard icIEF workflow. Figure 7 reflects the analysis of an upstream cell culture sample following Protein A purification. In this sample, acidic species increased in mass with a mass increment consistent with glycation, effectively masking lysine residues and showing how the acidic shift occurs. The basic species are most likely to be the result of lysine heterogeneity.
Process-related impurities can include everything from process components to HCP. Each impurity requires a unique approach for quantification and demonstration of its removal. The analytical development team at FDB Research Triangle Park (RTP) site has implemented methods for a wide variety of small molecule process components that can be used on triple quad mass spectrometers; evaporative light scattering detectors; or charged aerosol detectors, depending upon the sensitivity needed. The RTP site will serve as a center for process residual testing for small molecule components across the FDB network. FDB has also employed kit-based and molecule-specific HCP analysis for products made in a variety of cell lines, including CHO, E. coli, Sf9, pseudomonas, and Pichia. To characterize the coverage of these kits, FDB often collaborate with Cygnus to perform antibody affinity extraction and 2D differential gel electrophoresis. The team has also implemented an in-house procedure for evaluating HCP clearance and characterizing the HCP proteome via liquid chromatography mass spectrometry (LC-MS).
Devices to Implement High Throughput and Automation
Even with the depth of knowledge enabled by advanced characterization of protein structure, product-related species, and process-related impurities, it is sometimes critical to obtain a wide breadth of data about the product from extended characterization. In that regard, FDB implements high-throughput and automated workflows to allow scientists to focus on study design and data analysis rather than routine laboratory testing:
- The Big Kahuna automated workflow solution from Unchained Labs automates buffer exchange, forced degradation, and certain testing to enable higher throughput formulation development studies.
- The BioPhase 8800 capillary electrophoresis system from SCIEX allows FDB to test up to eight capillary electrophoresis sodium dodecyl sulfate (CE-SDS) samples at a time, which offers a nearly ten-fold increase in throughput of testing for formulation development or process characterization where multitudes of samples can be produced by those studies.
- The Waters Andrew+ pipetting robot is being evaluated to automate lengthy peptide mapping and glycan sample preparations.
- A collaboration between FDB RTP and FUJIFILM Wako Pure Chemical resulted in the development of a robotic testing system to fully automate testing of up to 24 samples per batch by UV, SEC-UPLC, icIEF, CE-SDS, and glycan profiling. After a single sample submission by an analyst, the Tecan liquid handler enables UV testing on an Unchained Labs Lunatic UV spectrophotometer. The custom scheduling software calculates sample preparations for each assay, with the Tecan then preparing the samples using reagents loaded by the analyst.
- A robotic arm built by FUJIFILM Wako Pure Chemical takes each plate from the Tecan and delivers it to the instrument for testing. The analyst monitors the data as it is generated and assesses the instruments if any issue arises.
Low endotoxin recovery (LER), the phenomenon in which purified endotoxin standards demonstrate poor recovery from product matrices, has become a challenge over the last decade. The hold studies to evaluate LER and the mitigation studies to improve recovery can be labor intensive. Therefore, FDB is investigating the use of an automated endotoxin testing system to reduce time needed to perform the studies needed to identify and mitigate LER effects in preparation for standard endotoxin testing through GMP manufacturing and process performance qualification.
The Bottom Line
Every protein has a unique personality that is based on structural and product-related characteristics as well as a process-related impurity profile. The evaluation of this personality using advancing analytical techniques not only provides the characterization data expected for regulatory submissions but also expands product knowledge to enable efficient and robust process development. FUJIFILM Diosynth Biotechnologies has the characterization tools to help understand the personalities of molecules and facilitate the product development cycle for our partners.
References
- Thomas, D., Chancellor, D., Micklus, A., LaFever, S., Hay, M., Chaudhuri, S., Bowden, R., & Lo, A. W. (2021). (rep.). Clinical Development Success Rates and Contributing Factors 2011-2020. Biotechnology Innovation Organization, Pharma Intelligence, and Quantitative Life Sciences. Retrieved from https://www.bio.org/clinical-development-success-rates-and-contributing-factors-2011-2020.
About the Author

Jason Barker is currently Director of Analytical Development, Analytical Method Transfer, and Formulation Development at FUJIFILM Diosynth Biotechnologies, Research Triangle Park, NC site. His group is responsible for the development, validation, and execution of analytical methods to support process and formulation development, characterization, and release testing of a wide variety of recombinant biotherapeutic candidates. Jason obtained his Bachelor of Science and Ph.D. degrees in Chemistry at North Carolina State University in Raleigh, NC and has 20 years of professional experience in biotechnology product development space.
About FUJIFILM Diosynth Biotechnologies
FUJIFILM Diosynth Biotechnologies, a subsidiary of FUJIFILM Corporation, is a world-leading contract development and manufacturing organization (CDMO) for the development and manufacture of biologics, advanced therapies, and vaccines. The company operates a global network with major locations in the Unites States of America, the United Kingdom and Denmark, offering end-to-end services including drug substance, drug product, and finished goods services. It is also building a new manufacturing site in Holly Springs, North Carolina, USA, scheduled to be operational in 2025. FUJIFILM Diosynth Biotechnologies has over thirty years of experience in developing and manufacturing drug substance of recombinant proteins, monoclonal antibodies, vaccines, among other large molecules, viral products and medical countermeasures expressed in a wide array of microbial, mammalian, and host/virus systems. We have drug product filling capabilities to support both clinical and commercial demands. Our finished goods services, supported by more than 15 years of experience, can accommodate commercial products for more than 65 countries around the world. The company offers a comprehensive list of services from cell line development using its proprietary pAVEway™ microbial and Apollo™X cell line systems to process development, analytical development, clinical and FDA-approved commercial manufacturing. For more information, go to: www.fujifilmdiosynth.com