Development of a Continuous Electroporation System for Viral Gene Therapy
Takashi Yakushiji
Process Engineering & Technology Center, FUJIFILM Corporation, Kaisei, Japan
Yoichi Nagai
Bioscience & Engineering Laboratories, FUJIFILM Corporation, Kaisei, Japan
The past three years have seen the approval of six adeno-associated virus (AAV) gene therapy products for rare genetic diseases, signifying growing confidence in the clinical efficacy of AAV in treating and curing previously difficult genetic diseases. With 1920 ongoing gene therapy clinical trials and a maturing pipeline of AAV, the stage is set for even bigger things to come1. While clinical validation has been successful, supporting future commercialization remains a challenge, as evidenced by the higher than anticipated dosage costs. Improving commercialization involves a variety of factors including reducing manufacturing costs and ensuring a robust supply of viral vectors. Contract development and manufacturing organizations (CDMOs) are well-positioned to contribute to these efforts.
The dosage requirements for viral gene therapies (VGTs) can vary greatly depending on the clinical indication, dose-efficacy requirements, size of clinical trials, and eventually, the size of patient populations. For targeted delivery, such as VGTs for the eye, dosages can range from 1E11-1E12 vector genomes (vg) per patient, while for systemic delivery into the liver or muscle tissues, as with Duchenne muscular dystrophy, higher dosages of 1E15-1E16 vg are required to account for the dilution of systemic administration2.
The development of VGTs requiring high-dose systemic delivery coupled with the goal of broadening VGTs to more prevalent indications like Parkinson’s or diabetes has created bottlenecks in the viral vector supply, which threatens to delay the delivery of novel therapies. Current PEI-mediated triple transfection manufacturing methods are limited by high cost, low productivity, and unproven scalability, making them unsuitable to satisfy the growing clinical and commercial demand3-5. Orders of magnitude higher productivity will drive lower cost of goods (COGs) and are a requirement to support the continued expansion of the field, which can only be achieved through optimized and scalable production systems.
The Current State of Viral Vector Manufacturing
Triple transfection is the most utilized production method to generate recombinant AAV (rAAV) gene therapy products today. This method involves co-transfection of three plasmids (the gene of interest (GOI) plasmid, the RepCap plasmid, and the helper plasmid) into mammalian cells (primarily HEK 293 cells) using polyethyleneimine (PEI) or other transfection reagents enabling the production of rAAV particles (Figure 1).
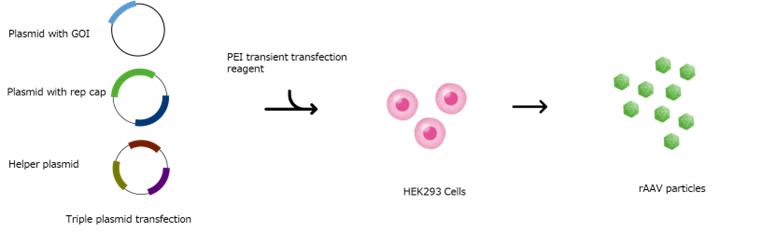
Conventional batch production of rAAV suffers from high costs and low volumetric yields, with average titers at approximately 1E11 vector genomes per mL (vg/mL)6. While this system offers versatility and allows for straightforward modification of the capsid and/or recombinant vector sequence, its scalability is limited due to the complexities of optimizing chemical reactions transfection reagent: plasmid complexing, stability of such complexes, and the dynamic interactions of those polyplexes and cellular interactions. The inception of the first commercial rAAV processes utilized adherent systems, and while effective for some applications, the industry has since moved to low-density suspension-based processes to scale-up as opposed to scale-out. To further improve suspension-based production, protocols have continued to be refined and improved, incorporating new types of technology to increase productivity; however, a proverbial ceiling exists which restricts production scales and limits total vector output. Rather than continuing to pursue scale up, manufacturers have begun adopting a strategy of scaling out suspension production, where multiple batches are produced at a manageable scale to generate large quantities of rAAV for treating large, systemic indications. However, this shift has created a bottleneck in production capacity and suggests the need for alternative methodologies to overcome the existing production ceiling.
One potentially disruptive methodology eliminates the need for chemical transfection reagents altogether. Electroporation, a widely utilized physical method for transfecting cells with various molecules, offers a promising alternative to PEI-mediated transient transfection. By subjecting cells to high-voltage electrical pulses, transient pores are created in the cell membrane through which plasmid DNA can diffuse into the cells. However, standard static cuvette-based approaches have their own limitations, including extensive and difficult optimization processes, low throughput, and poor cell viability, which is incompatible with large-scale manufacturing7. While batch-type electroporation systems are available, they fail to meet the productivity and throughput demands for viral vector production.
Development of a Novel, Continuous Electroporation System
To address these limitations, the Fujifilm Bio Science & Engineering Laboratories (BSEL) has developed an innovative and scalable, chemical-free continuous viral vector production platform. The platform can effectively scale up an established transient expression technology by combining high-density HEK293 perfusion cell culture with high-throughput continuous transfection using a novel continuous electroporation (continuous EP) system. A development timeline for the continuous EP system is shown in Figure 2.
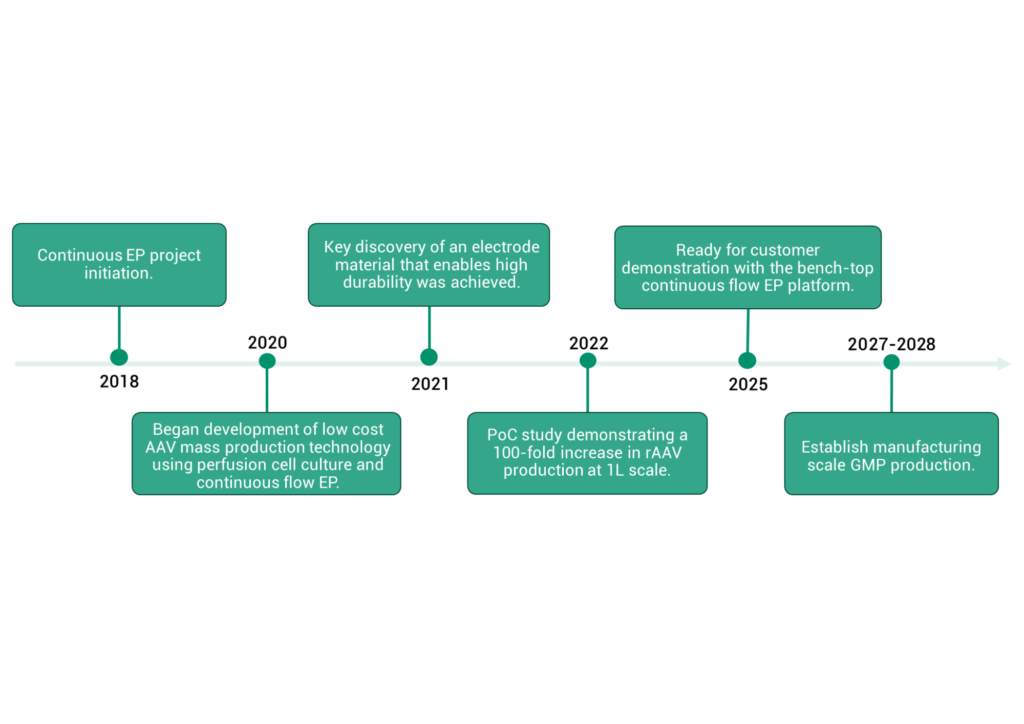
Here, we describe the development work involved for the individual technologies that make up the continuous rAAV production platform.
High-Density Cell Culture Optimization
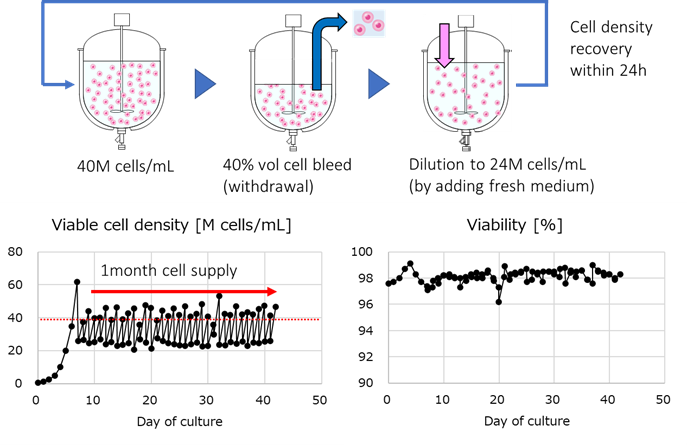
The BSEL team first conducted process development to optimize conditions for a 1L-scale Alternating Tangential Flow (ATF)-based semi-continuous HEK293 perfusion process to achieve a target cell density of 40 million (M) cells/mL and high cell viability over a 1-month culture period (Figure 3).
Continuous EP instrument Development
Commercially available electroporation (EP) systems lack key characteristics for mass-producing adeno-associated virus (AAV) in terms of both durability and throughput. Therefore, BSEL – in collaboration with Fujifilm’s Process Engineering & Technology Center (PETC) – embarked on the development and production of a scalable continuous EP instrument to overcome these limitations (Figure 4).
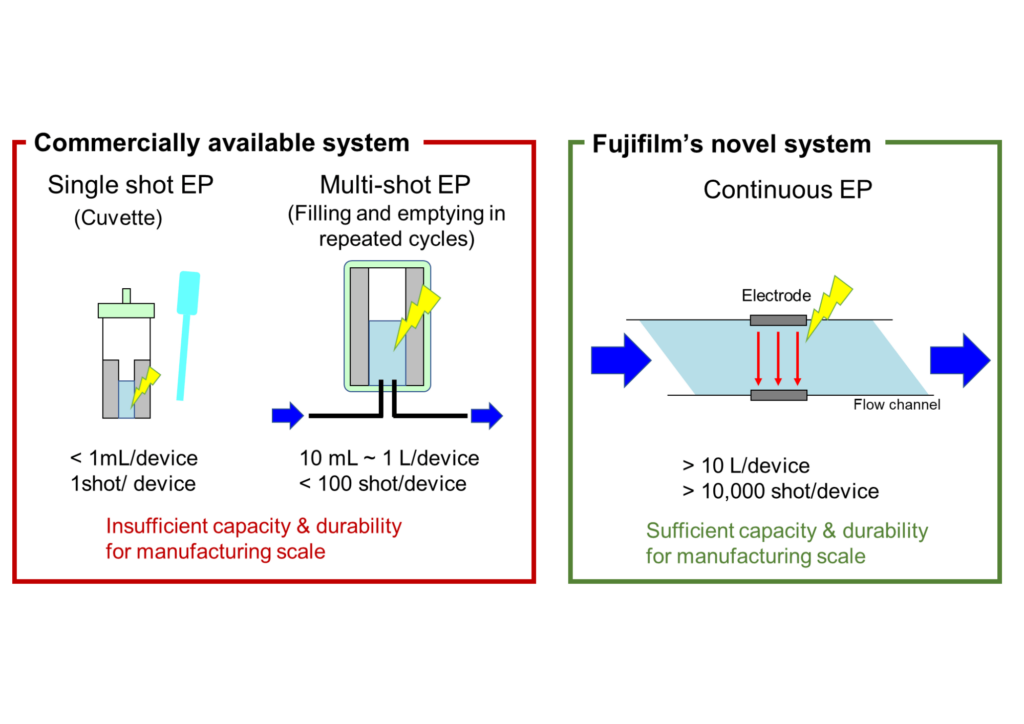
The continuous EP instrument offers several key advantages for efficient gene transfection. Firstly, the single-use electrode unit is highly durable, ensuring consistent and reliable delivery of many thousands of repeat electrical pulses over multiple cycles without degradation (> 10,000 shot pulses for > 10 L batch volumes). Additionally, the instrument features an optimized flow channel structure and pulse conditions for high throughput, high-density cell suspensions with optimal transfection efficiency. The continuous electroporation process can facilitate rapid and high-throughput plasmid introduction (~ 100 mL/min) as compared to static (~ 10 µL/min) or batch EP (1-10 mL/min) methods.
Proof of Concept Study
Following the successful development of the perfusion and continuous EP technologies, the two processes were integrated aseptically, and a 1-month continuous proof-of-concept (PoC) trial was conducted at the BSEL laboratories. A schematic of the workflow is shown in Figure 5.
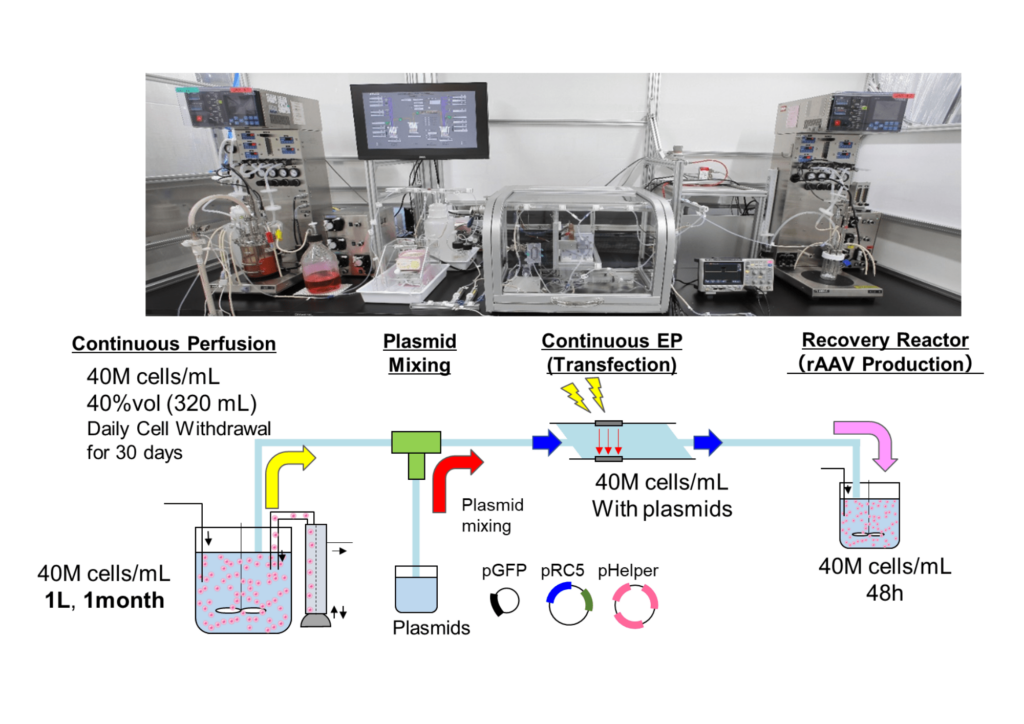
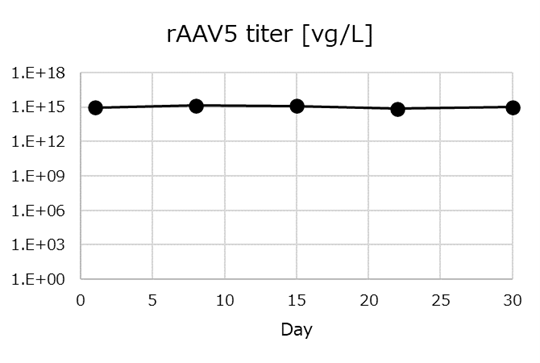
Throughout the experiment, cells were continuously maintained in culture at a concentration of 40M cells/mL in a 1 L bioreactor with 40% of the bioreactor volume withdrawn each day. Plasmid DNA was introduced into the cells using continuous EP once per week and transferred to a recovery bioreactor for rAAV5 production. rAAV titer was quantified 48 hours after each round of plasmid introduction. An average titer of 1E15 vg/L was successfully maintained throughout the experiment (Figure 6).
Additionally, the PoC study successfully confirmed the durability of the single-use electrode throughout the entire duration of the 1-month experiment. The data generated from the PoC experiment was compared to the performance of a conventional batch PEI transfection method to determine the overall improvements achieved through the new approach (Table 1).
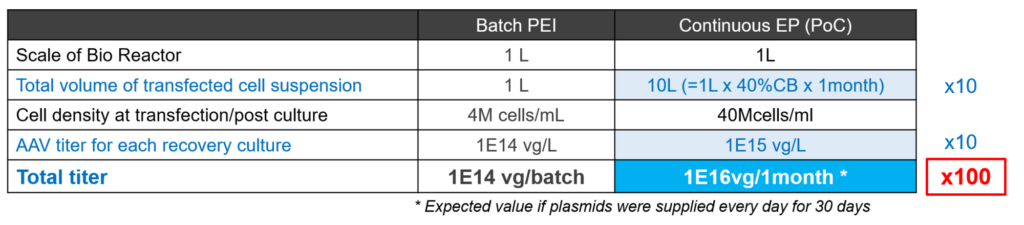
Through the developed continuous perfusion method, 10X greater cell suspension volumes and a 10-fold increase in titer for each post-culture sample was achieved compared to conventional batch approaches. Although plasmid introduction was limited to once a week for the PoC experiment, theoretical calculations suggest that supplying plasmid DNA on a daily basis could yield a total rAAV titer of 1E16 vg from a 1 L production bioreactor. This represents a productivity increase of 100-fold compared to the equivalent bioreactor scale with batch-mode, traditional manufacturing cadence and chemical triple transfection production. This innovative platform has demonstrated its ability to effectively scale up established transient expression technology for viral vectors, which can facilitate the rapid adoption and migration of products made with conventional approaches.
Conclusion
The chemical-free continuous rAAV viral vector production platform described here has the potential to dramatically increase rAAV productivity by the orders of magnitude needed to solve current viral vector manufacturing bottlenecks. The scalable platform can accelerate clinical development by reducing time-to-product from many months to just a few weeks, and expand commercialization of rAAV-based gene therapies for a wider range of diseases. The continuous EP instrument is anticipated to be ready for laboratory demonstrations with our industry partners in early 2025.
In the near-term, ongoing development efforts will focus on downstream quality attribute evaluation to demonstrate the equivalency of continuous EP-generated rAAVs, with respect to potency, with those produced from validated chemical-based production platforms. Partnerships with clinical-stage biotechnology companies will center on these critical process attributes, utilizing standard analytical testing methods to demonstrate comparability profiles. Additionally, development is currently underway to establish manufacturing scale GMP production.
While transient transfection offers an immediate and shortened solution for early-phase clinical trials, the BSEL team, located in Fujifilm’s Advanced Research Laboratories, Kaisei, Japan (Figures 7 and 8) are actively engaged in ongoing efforts to enhance rAAV production through the development of stable producer cell lines. This initiative aims to improve vector productivity, streamline manufacturing processes, and reduce costs, to help make VGTs more economically viable and accessible on a broader scale. These and other innovation projects at BSEL exemplify Fujifilm’s commitment to support the collective advancement of the field for the betterment of patients worldwide.


References
- Alliance for Regenerative Medicine (April 2024). Sector Snapshot: Advances in Engineered Cell Therapy. https://alliancerm.org/sector-snapshot-april-2024/
- Au, H. K. E., Isalan, M., & Mielcarek, M. (2022). Gene Therapy Advances: A Meta-Analysis of AAV Usage in Clinical Settings. Frontiers in medicine, 8, 809118. https://doi.org/10.3389/fmed.2021.809118
- Smith, J., Grieger, J., Samulski, J. (2018). Overcoming Bottlenecks in AAV Manufacturing for Gene Therapy. Cell Gene Therapy Insights 2018; 4(8), 815-827. https://doi.org/10.18609/cgti.2018.083
- Merten, OW (2016). AAV vector production: state of the art developments and remaining challenges. Cell Gene Therapy Insights; 2(5), 521-551. https://doi.org/10.18609/cgti.2016.067
- Fu, Q., Polanco, A., Lee, Y. S., & Yoon, S. (2023). Critical challenges and advances in recombinant adeno-associated virus (rAAV) biomanufacturing. Biotechnology and bioengineering, 120(9), 2601–2621. https://doi.org/10.1002/bit.28412
- Hebben, M (2018). Downstream bioprocessing of AAV vectors: industrial challenges & regulatory requirements. Cell Gene Therapy Insights; 4(2), 131-146. https://doi.org/10.18609/cgti.2018.016
- VanderBurgh, J. A., Corso, T. N., Levy, S. L., & Craighead, H. G. (2023). Scalable continuous-flow electroporation platform enabling T cell transfection for cellular therapy manufacturing. Scientific reports, 13(1), 6857. https://doi.org/10.1038/s41598-023-33941-2
About the Authors

Takashi Yakushiji
Process Engineering & Technology Center, FUJIFILM Corporation
Takashi Yakushiji is a Senior Process Engineer at the Process Engineering & Technology Center of FUJIFILM Corporation in Japan. With 15 years of experience at Fujiflm, he has worked in product development across various fields such as semiconductor materials, functional films, and the inkjet business, specializing in microfabrication processes. In 2020, Takashi transitioned to the field of biotechnology, where he utilizes his extensive experience and expertise to advance technologies for gene therapy. Currently, he is responsible for the development of Continuous Electroporation technology, which aims to establish a leading-edge platform for high-yield and continuous production of adeno-associated virus (AAV). Takashi is actively involved in all aspects of Continuous Electroporation technology, including research, process development, demonstration, and the development of equipment compliant with cGMP. He holds a Master’s Degree in Electronics Engineering from Kyoto University.

Yoichi Nagai
Bioscience & Engineering Laboratories, FUJIFILM Corporation
GT Development Team Leader
Yoichi Nagai has been engaged in the development of production processes for pharmaceuticals and functional industrial materials for 30 years at Fujifilm, specializing in membrane separation process design. In 2012, he moved into the field of biotechnology, developing perfusion culture processes for antibodies, and in 2018, he established a project team to continuously produce adeno-associated viruses (AAV) in high yield. He is currently the project team leader. He holds a Master’s degree in Materials Science and Engineering from Yokohama National University.