An AAV Platform Advancing Successful Viral Vector Based Therapeutics
By Michael Baker, PhD, Senior Director, Viral Gene Therapy
Increasing demand for viral vector production
Decades of technological innovation and scientific advances have revolutionized the treatment paradigm for many conditions that have long eluded conventional therapies.[i] At the forefront of these innovative modalities, advanced viral vector-based therapeutic and immunization strategies such as gene therapy, oncolytic viruses, and viral vaccines offer the promise of preventing or slowing the progression of, and perhaps even curing, previously unreachable conditions.[ii]
Despite getting off to a rocky start,[iii],[iv],[v] viral vectors are experiencing a renaissance in both preclinical and clinical applications. No longer limited to well-understood monogenic diseases, next-generation viral gene therapies (VGTs) and disease prevention strategies can dramatically expand the potential to prevent and treat a wide spectrum of diseases, bringing hope to patients suffering from both rare and broad-population diseases including diabetes, degenerative diseases, HIV/AIDS, and specific cancers.1,[vi],[vii]
As excitement over VGTs continues to grow, so does the demand for viral vector production. More than 20 cell and gene therapies have received FDA approval since 2017,1,[viii] and it is expected that this rate will increase to 10-20 per year by 2025.2,[ix] The shift beyond rare and ultrarare to broader indications for viral-based therapies and the increased demand for viral vector vaccines brought about by the COVID-19 pandemic continue to exacerbate this capacity crunch, adding to other significant constraints in large-scale viral vector manufacture.
Addressing substantial challenges in viral vector manufacture
Viral vector development is littered with obstacles at virtually every stage. The lack of universal, “one-size-fits-all” production systems and standardized upstream and downstream processes poses challenges to both emerging biotech companies and large pharma developers. In addition, the development of assays to assess quality attributes can be time and cost intensive, further contributing to delays in timelines and increased costs.
Although scalability can be a concern for early-stage developers, viral-based therapy development is often hampered by tight timelines and funding mechanisms that require feasibility studies, as well as a lack of access to technology and raw materials. In contrast, large pharmaceutical companies may face hurdles related to a dearth of expertise, because of over reliance on tried and tested production platforms creating a gap in advanced therapy development experience. In addition, most developers typically pursue multiple different indications, and therefore need an approach that allows for the flexibility and agility to reprioritize candidates at short notice.
The use of a state-of-the-art, customizable AAV platform that incorporates streamlined processes and access to ‘ready-to-go’ critical materials can circumvent time, cost, and raw material access constraints that accompany viral vector manufacture and help propel these therapies from the lab to clinic to market.
A platform Approach – the FUJIFILM Diosynth Biotechnologies’ advantage
FUJIFILM Diosynth Biotechnologies is an industry leading CDMO with 30+ years’ experience in process development and GMP manufacturing of viral vectors for gene therapy and viral vaccine products. Our expert team has successfully executed 80+ projects that encompass a broad range of viruses and vector types, including AAV, AdV, and LV, among others.
Our AAV platform approach drives rapid and predictable progression of high-quality viral vector products from process development to clinical and commercial supply, and the harmonized design of our state-of-the-art facilities in the US (College Station, TX) and UK (Darlington) allows for increased flexibility in the choice of scale and location. We also offer drug product and fill finish services for VGT products.
FUJIFILM Diosynth Biotechnologies’ AAV platform approach overcomes key challenges in viral vector manufacture by offering access to a commercial-ready cell line, plasmids, and technology, increasing scalability and reducing timelines and costs. Our cell culture experts and virologists have developed and optimized a cGMP clonal suspension HEK293 cell line for consistently high rAAV titers across multiple serotypes and scales. Full to empty capsid ratios average ~25% at harvest and >70% upon purification.
Our transfection system results in consistent and high rAAV titers for multiple serotypes and at multiple scales.
In addition, our flexible approach allows for the use of non-platform plasmids without impacting product titer or bioreactor performance and can be applied to subsequent GOI constructs with minimal development, allowing for the pursuit of multiple different indications at lower cost and under reduced timelines. We offer an off-the-shelf, ‘pay-as-you-use’ GMP-grade plasmid toolbox that circumvents the need to pay for dedicated cell bank and plasmid manufacture, contributing to predictable and manageable costs.
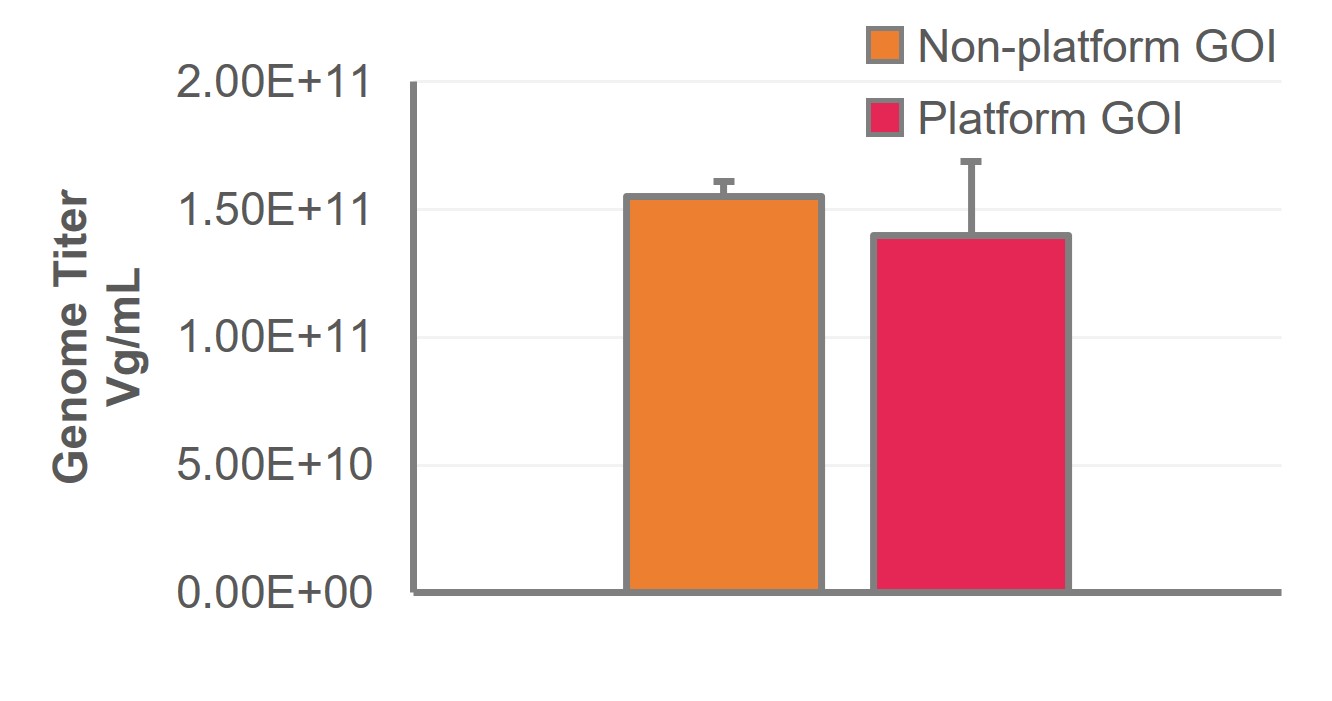
Comparable titers observed at harvest when using platform or non-platform GOI plasmids.
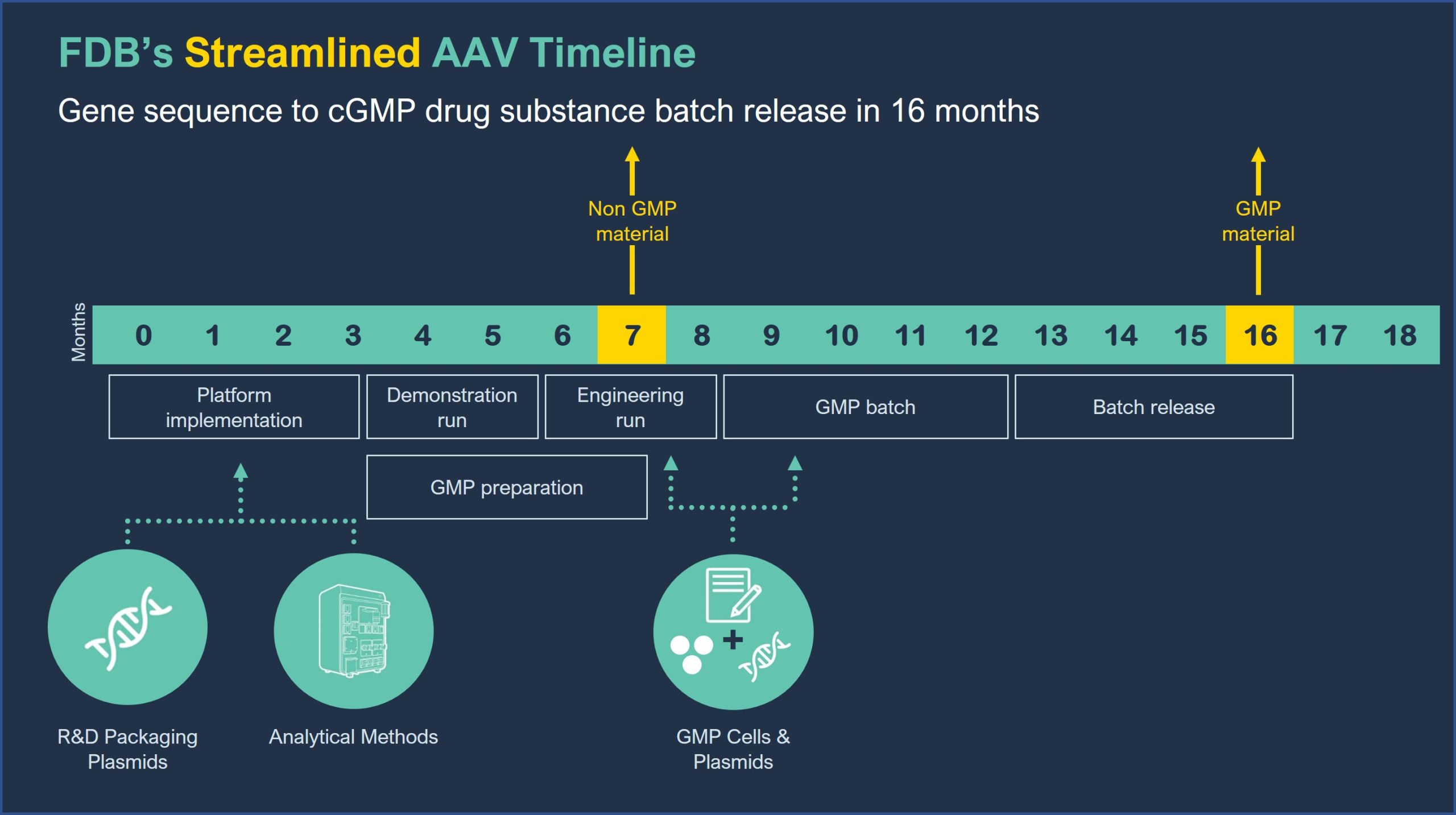
FUJIFILM Diosynth Biotechnologies’ AAV platform offers processes and analytical methods that are applicable to most AAV serotypes and minimizes the need for lengthy process development, allowing for flexibility to reprioritize candidates at short notice. Access to our cGMP cell lines and plasmids allows gene to cGMP batch release within 16 months, while non GMP material can be available in 7 months.
Your advanced therapies CDMO partner for life
REFERENCES
[1] Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021. Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 6(53), PMID: 33558455, https://doi.org/10.1038/s41392-021-00487-6.
[2] Lundstrom K. 2018. Viral Vectors in Gene Therapy. Diseases 6(2):42, PMID: 29883422, https://doi.org/10.3390/diseases6020042.
[3] Raper SE, Chirmule N, Lee FS, Wivel SA, Bagg A, Gao GP, Wilson JM, Batshaw ML. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab 80:148–158, PMID: 14567964, https://doi.org/10.1016/j.ymgme.2003.08.016.
[4]McCormack MP, Rabbitts TH. 2004. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 350:913–922, PMID: 14985489, https://doi.org/10.1056/NEJMra032207.
[5]Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, et al. 2008. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Investig 118:3132–3142, PMID: 18688285, https://doi.org/10.1172/JCI35700.
[6] Shahryari A, Saghaeian JM, Mohammadi S, Razavi NH, Nazari Z, et al. 2021. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front Genet 10(868), PMID: 31608113, https://doi.org/10.3389/fgene.2019.00868.
[7] Papanikolaou E, Bosio A. 2021. The Promise and the Hope of Gene Therapy. Front Genome Ed 3(618346), PMID: 34713249, https://doi.org/10.3389/fgeed.2021.618346.
[8] U.S. Food & Drug Administration. 2021. Approved Cellular and Gene Therapy Products. Silver Spring, MD: U.S. Food & Drug Administration. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products [accessed 22 Feb 2022].
[9] U.S. Food & Drug Administration. 2019. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. Silver Spring, MD: U.S. Food & Drug Administration. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics [accessed 18 Feb 2022].