A Look Inside Fujifilm’s Innovation Hub
Situated in Kaisei, Kanagawa Prefecture, located 80 km outside of Tokyo, Fujifilm’s Advanced Research Laboratories (Figure 1) is an innovation hub and core research and development institute. It is organized with various research divisions such as the Bioscience and Engineering Laboratory (BSEL), which focuses on the biological research field, and the Process Engineering & Technology Center (PETC), which specializes in process technologies to enable optimal manufacturing. Drawing upon a wealth of expertise spanning diverse disciplines such as imaging and image processing, materials sciences, and life sciences, researchers at Kaisei are dedicated to pioneering new and differentiated technologies with an eye to the future. The collaborative atmosphere and melting pot of talent encourages interdisciplinary knowledge sharing to address complex challenges and develop innovative solutions.

At BSEL, our R&D initiatives leverage the fundamental technologies and corporate knowledge that Fujifilm has nurtured over many years to support the goals of the organization in the biomedical area including protein manufacture, gene therapy, cell therapy and regenerative medicine. These initiatives utilize our core competencies in process development and manufacturing to improve mass production technologies for various biopharmaceuticals to help bring new products and services to market that will drive the industry forward and broaden patient access to novel therapeutics on a global scale.
In this article, we will highlight two projects that exemplify the scope and diversity of our ongoing research and technical innovations in Kaisei. The overarching theme across these projects is a focus on improving efficiency and productivity in biopharmaceutical manufacturing while also reducing the cost of goods through process intensification.
Development of a Continuous Electroporation System for Viral Gene Therapy
One of the biggest bottlenecks in expanding the application of viral gene therapies (VGT) into more common indications is the high cost and low productivity of current PEI-mediated triple transfection manufacturing methods. For high prevalence indications, such as arthritis or diabetes, current production systems will not be able to generate the quantity of viral vector at the low cost required for treatments.
To overcome current limitations, the BSEL group has pioneered a truly scalable chemical-free continuous rAAV viral vector production platform. This intensified process combines high-density HEK293 perfusion cell culture with high-throughput continuous transfection using a novel continuous electroporation (continuous EP) developed and built at Kaisei (Figure 2). This scalable platform can dramatically increase rAAV productivity by orders of magnitude (≥ 100 fold), accelerating clinical development and opening up commercialization of rAAV-based gene therapies for a wider range of diseases.
During the instrument’s development, a key challenge was identifying a suitable electrode material with the strength and durability to withstand the many thousands of repeat pulses needed for continuous EP. This required cross-functional collaboration across Kaisei, including PETC’s experts in the surface treatment and the electronics development, in addition to the bioscientists in BSEL, to fabricate and test electrodes that could meet all the necessary requirements.
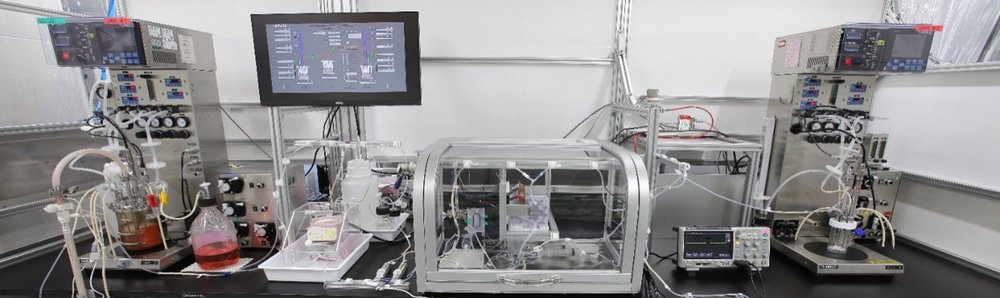
An instrument for cutomer demonstration is anticipated to be ready before the end of 2024, with an upcoming companion article that will provide details on the development and capabilities of the continuous EP system as well as showcase the proof of concept data.
Further Simplifying Viral Vector Production
While the continuous EP system can effectively scale up an established transient expression technology for viral vectors to allow for rapid adoption and migration of products made with conventional approaches, it still requires significant quantities of plasmid DNA, which can be expensive to produce and sometime difficult to source at GMP quality.
To move further towards our goal of conquering viral vector mass production and driving down production costs, the BSEL team have an advanced cell line development project to use inducible, plasmid transfection free, producer HEK293 cells. By engineering these stable producer cell lines with an inducible expression system, we can precisely control the activation of AAV vector production and achieve higher and more consistent productivity. Leveraging stable cell lines also allows us to eliminate the need for costly plasmid reagents and remove the remaining challenges associated with scaling up transient transfection for large-scale production.
Implementing stable producer cell lines not only enhances vector productivity but also streamlines the manufacturing workflow, which will help reduce overall manufacturing costs and help make VGTs more economically viable and accessible to a broader patient population. Our AAV producer cell lines are expected to enter the testing phase with FUJIFILM Diosynth Biotechnologies and selected test clients.
Shaping the Future of Bioprocessing
At its core, BSEL embraces a culture of innovation and continuous improvement that supports Fujifilm’s business initiatives while also positively impacting the bioprocessing industry as a whole. Whether its evaluating new technologies or designing better processes, the life sciences team (Figure 3) consistently pushes the boundaries of biomanufacturing in pursuit of innovations like the example projects we’ve described here. These initiatives not only address current needs but also anticipate future challenges and opportunities in a fast-paced industry. With a forward-looking approach, BSEL continues to drive bioprocessing advancements today that will enable faster and more affordable delivery of life-changing medicines to ensure a brighter future for patients worldwide.

About the Authors

Figure 3. Bioscience and Engineering Laboratory and Process Engineering & Technology Center team members
Takashi Yakushiji
Process Engineering & Technology Center, FUJIFILM Corporation
Takashi Yakushiji is a Senior Process Engineer at the Process Engineering & Technology Center of FUJIFILM Corporation in Japan. With 15 years of experience at Fujiflm, he has worked in product development across various fields such as semiconductor materials, functional films, and the inkjet business, specializing in microfabrication processes. In 2020, Takashi transitioned to the field of biotechnology, where he utilizes his extensive experience and expertise to advance technologies for gene therapy. Currently, he is responsible for the development of Continuous Electroporation technology, which aims to establish a leading-edge platform for high-yield and continuous production of adeno-associated virus (AAV). Takashi is actively involved in all aspects of Continuous Electroporation technology, including research, process development, demonstration, and the development of equipment compliant with cGMP. He holds a Master’s Degree in Electronics Engineering from Kyoto University.

Yusuke Mori
Bioscience & Engineering Laboratories, FUJIFILM Corporation
Yusuke Mori is a researcher at Bioscience & Engineering Laboratories of FUJIFILM Corporation in Japan. Since 2016, he has been conducting research on various types of cells based on molecular biology. Currently, he focuses on cells that produce adeno-associated virus (AAV) used in gene therapy and is working on the development of next-generation virus production methods. By editing the cell genome, he has established stable cell lines that can induce virus production with a small molecule and developed a highly productive and scalable production method. He received his Doctor of Science degree from Waseda University.