Emerging Vaccine Technologies
Rapidly shifting modalities require broad CDMO expertise and capacity
You will recall from our previous installment, The Changing Vaccine Development Landscape, protein subunits and recombinant vectors now dominate the preclinical and clinical infectious disease and oncology vaccines pipeline.
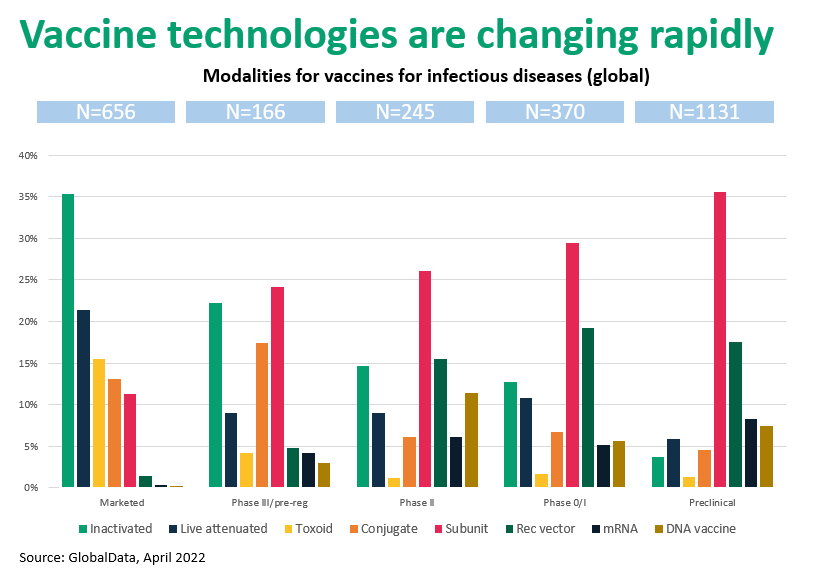
Figure 1. Vaccine technologies are rapidly changing from “live” formats to “molecular” vaccines
To summarize:
- Marketed vaccine types include inactivated organisms, toxoid, conjugate, protein subunits, and genetic (mRNA, DNA), each with unique development and manufacturing requirements.
- Early clinical and preclinical phases are dominated by subunit and recombinant vaccines.
Subunit and Recombinant Vector Vaccines
Live-attenuated and inactivated vaccines use weakened or “dead” versions of the disease-causing microorganisms (typically viruses), respectively, to induce an immune response. Live-attenuated products are contraindicated in immunocompromised individuals and in pregnant women. Inactivated vaccines generate less-robust immunity and require boosters, and both vaccine types require low-temperature storage. While these traditional vaccine modalities are still relevant, their practical limitations, plus the emergence of exciting new vaccine technologies (and their potential to prevent or treat non-infectious diseases like cancer), are behind the shift from organism-based to molecular vaccines.
Subunit vaccines are based on molecular features of the pathogen, for example an antigenic region on a surface protein or some other proteomic feature. These vaccine types confer strong immunity and are generally safer for patients with weakened immune systems or comorbidities, but they also often require boosters. Among these vaccines are products that immunize against Haemophilus influenzae type b, hepatitis B, human papillomavirus, whooping cough, pneumococcal disease, meningococcal disease, and shingles.
Subunit vaccines represent an interesting and diverse grouping that includes recombinant proteins, virus-like particles, and synthetic peptides. Approximately 75% of subunit vaccines are produced recombinantly. More than 2/3 of these use E. coli, with the remainder using yeasts such as P. pastoris, or baculovirus expression systems. Compared with vaccines based on more-or-less intact pathogens, subunit vaccines are non-infectious since they incorporate only antigenic components of disease causing bacteria, parasites, or viruses.
Developers of subunit vaccines face issues similar to those encountered in the commercialization of therapeutic proteins: cell line and clone selection, media optimization, quality, purification, and scaleup. Subunit vaccines are considered the least-risky immunization technology, but these products often require adjuvants to enhance their effectiveness. Developers must also navigate a complex matrix of antigen, modifications to the peptide epitope, and delivery mechanism, although batch sizes tend to be much smaller for vaccines generally than for proteins
Recombinant vector vaccines are live, replicating, innocuous organisms engineered to carry genes coding for specific antigen(s) on the pathogen (bacterium, virus, or yeast). Because these vaccines act like a natural infection they stimulate a wide range of beneficial immune responses, but they may not be the best choice for individuals who are very sick or with compromised immune systems. The COVID-19 vaccines from Astra-Zeneca and Johnson & Johnson use adenovirus as the vector, but other viruses are also used.
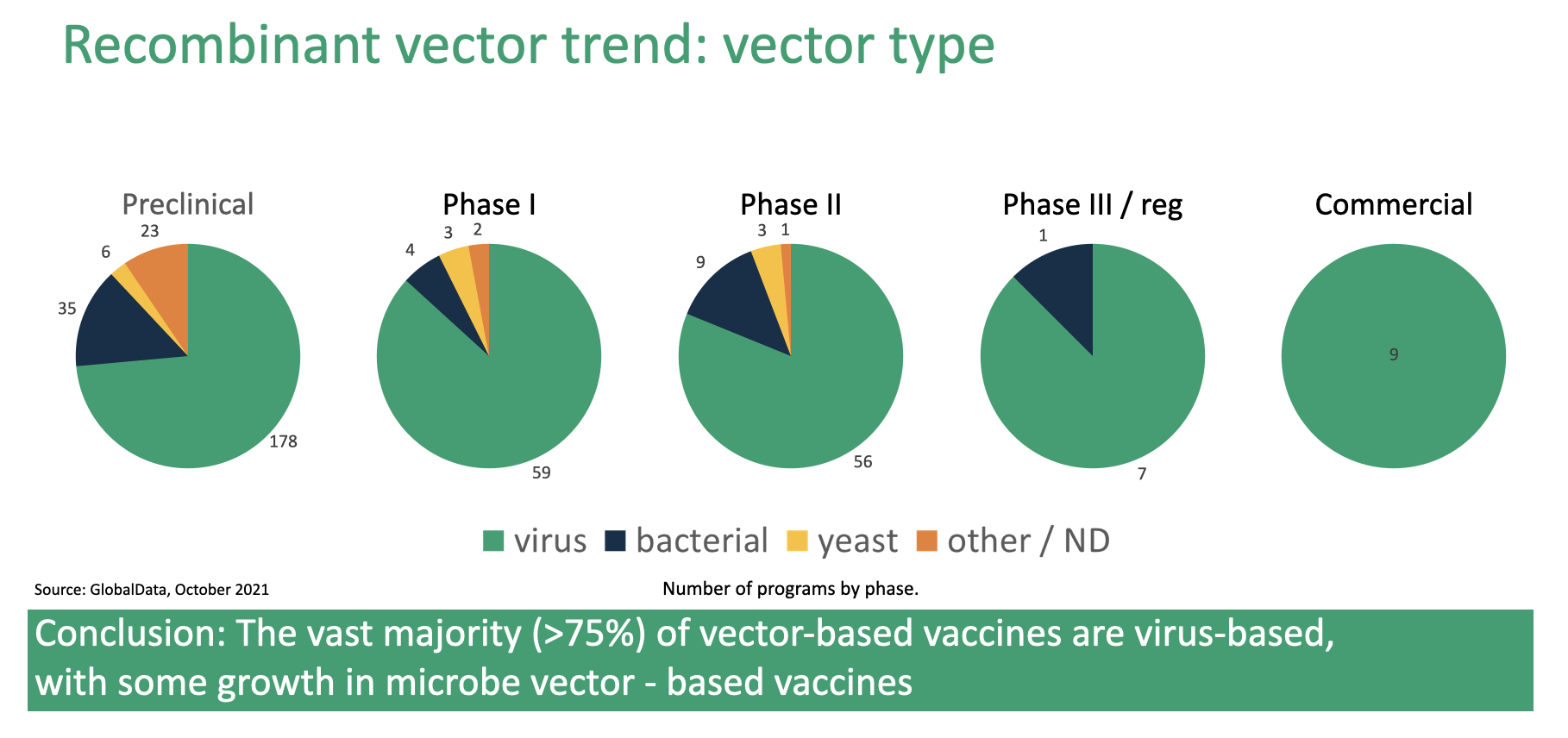
Figure 2. Expression systems used to develop recombinant vector vaccines
Figure 2. shows the distribution of pathogens, by development stage, used to create recombinant vector vaccines. Here we see a trend like that illustrated in Figure 1., where all commercialized vaccines use viral vectors but bacterial, yeast, and “other” vectors make up an increasing share as one moves to earlier stages of development.

Figure 3. Five virus types cover majority of the recombinant vector vaccine landscape
Figure 3. shows that despite the emergence of bacterial, yeast, and “other” vectors, viral vectors will dominate recombinant vector vaccine development for many years.
Commercializing recombinant vector vaccines requires a skill set that differs from protein or peptide based approaches. Figure 3., which lists the viruses utilized by development stage, shows complete dominance by adenovirus with vaccinia comprising about 20% of products. Although adenovirus continues to dominate in early clinical stages, we see substantial utilization of lentivirus, vesicular stomatitis virus, and others. A CDMO should be prepared to support the client’s vector selection or to suggest one from their own portfolio. FUJIFILM Diosynth Biotechnologies has broad and deep experience with virus and viral vector production, plus the analytical expertise to ensure drug product quality and potency at a range of production scales.
Although these viruses are selected for low pathogenicity (and most are non-pathogenic) they are still infectious, which requires maintaining development facilities at a high biological safety level. Viruses may require genetic engineering to reduce or eliminate pathogenicity. Also, most viral vectors are replication-defective, which makes them harder to scale up. In adenovirus vectors, for example, genetic regions required for replication are deleted and replaced with the therapeutic gene.
Finally, virus production has inherent scalability issues. Host cells cannot be grown to the same density as, say, Chinese hamster ovary cells for monoclonal antibody production. Creating banks for host cells is complicated because the viral construct can kill the cell. On the positive side since vaccines are highly potent products and production volumes tend to be much smaller than for therapeutic proteins.
Oncology vaccines prevent and treat cancers. Although prophylactic vaccines against cancer-causing viruses (e.g., papillomavirus, hepatitis) are often categorized as cancer vaccines, they are infectious disease vaccines. The FDA has approved three therapeutic vaccines that treat cancer directly: BCG live (for treating early stage bladder cancer), Sipuleucel-T (prostate cancer), and talimogene laherparepvec (melanoma). Ongoing clinical studies[1] are testing dozens of other oncology vaccines to treat a wide range of cancers.
Experience Matters
As developers shift from organism-based vaccines for “molecular” products, the technologies underlying the development and manufacture of these products are rapidly changing. With experience in both viral vector and subunit approaches, FUJIFILM Diosynth Biotechnologies is uniquely positioned to support the commercialization of today’s crop of development-stage vaccines to meet existing and emerging medical needs.
With more than 30 years of experience in microbe and virus based cGMP biomanufacturing, FUJIFILM Diosynth Biotechnologies can assist with the development and production of subunit, inactivated/live attenuated, and recombinant vector vaccines. Our experience ranges from expression to purification and the manufacture of drug product, from preclinical to clinical supply, to post-approval.
CDMO Partner for Life
With broad and deep expertise in vaccines development and a global manufacturing network, we provide competitive advantage and business value.
Contact us to learn how we can accelerate your vaccine development and commercialization.
REFERENCES
[1] Skwarczynski M, Chandrudu S, Rigau-Planella B, Islam MT, Cheong YS, Liu G, Wang X, Toth I, Hussein WM. Progress in the Development of Subunit Vaccines against Malaria. Vaccines (Basel). 2020 Jul 10;8(3):373. doi: 10.3390/vaccines8030373. PMID: 32664421; PMCID: PMC7563759.