Monoclonal Antibody Platform: mAb Platform

Fast-track Your mAb to Toxicology and IND
Our high-yielding gene-to-IND monoclonal antibody (mAb) platform – coordinated by a team of dedicated experts – offers a rapid, predictable timeline and costs. Designed for customers that want to prioritize speed to both toxicology studies and IND, it is ideal for standard mAbs, yet fully able to accommodate some novel antibodies.
Why Compromise on Reliability and Robustness for Speed When You Can Have Both?
Prompt start – Your program can start right away with off-the-shelf consumables, a standardized bill of materials combined with platform process and analytical methods.
No need to wait for another campaign to finish – Once you enroll in our mAb platform program, we will build clinical and commercial slots into the schedule, further increasing the predictability of your timelines and material supply.
Gene to GMP in 13 months – We take you from gene to GMP in 13 months thanks to the rapid implementation of modular, parallel workflows for cell line, process development, CMC, drug substance and drug product. Options for accelerating this timeline even further can be discussed with our subject matter experts.
Tox material within 6 months – If you are looking to gain maximum insight on your candidate molecule as early as possible, we can provide toxicology material within 6 months from program initiation to enable you to perform toxicological studies before entering cGMP manufacturing.
Platform process and analytical methods – Every mAb presents unique characteristics. As such, core platform analytical methods are refined through Quality by Design (QbD) systems and predictive software. This ensures the Critical Quality Attributes (CQAs) are monitored and controlled in readiness for cGMP.
Data you need for IND submission – Our team of experts are well versed in creating documentation containing all the required data from a CDMO to support your IND filing.
Predictable product yields – Rest easy knowing you can expect on average 2 kg of purified antibody from an Apollo X manufacturing run.
A dedicated team of mAb platform experts – Having a dedicated mAb platform team means we have experts who work with customers like you day in and day out and can anticipate your needs. They are able to leverage learning from a wide range of mAbs and novel antibody formats, building extensive experience in production and processing. For more challenging mAbs, our teams are ready with a working toolbox of solutions to use to ensure your program’s progress.

mAb Platform and Apollo X: A Tried-and-True pair
Our mAb platform was developed to process the high titers of Apollo X-expressed molecules. After successfully expressing your monoclonal antibodies via our mammalian expression system, we recommend our mAb platform so you are assured 2 kg of purified antibody on average.

Proven Ability to Accommodate Other Cell Lines
We recognize that some developers may have already made a start with their cell line when they approach us. If you already have a cell line, we can seamlessly technology transfer it into our mAb platform as a gateway to our network.
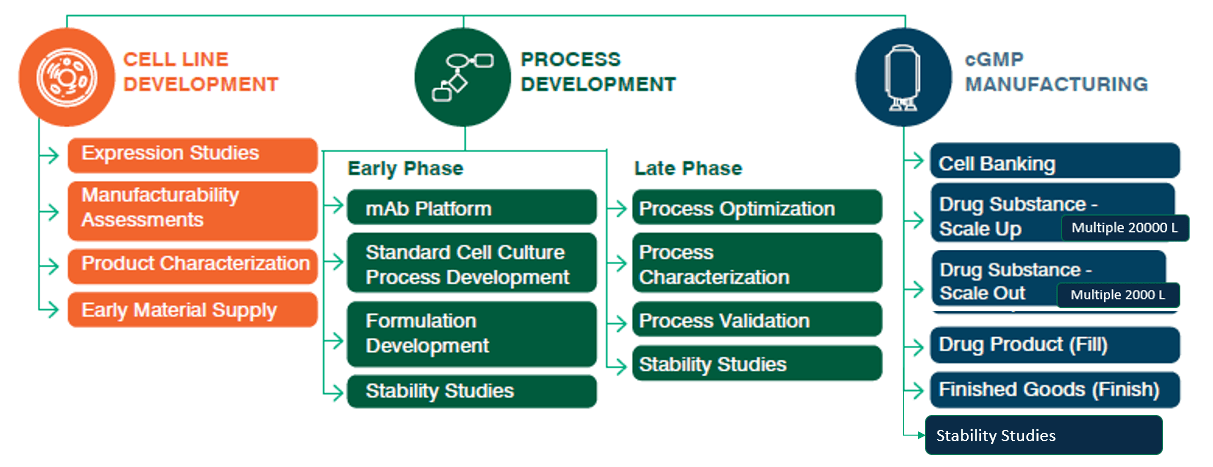
Go Further
Choose a CDMO that will set you up for success in the long run and be a true partner on your journey to commercialization. Explore our manufacturing strategies:

Discuss your project