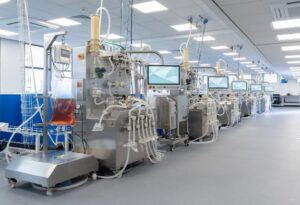
MaruX™: Fully Integrated Continuous Manufacturing Process
An agile, fully integrated continuous processing platform
With our high-productivity and single-use technology, we provide you with a continuous manufacturing approach with easy-to-modulate capacity and efficient end-to-end sustainable processes. MaruX, our fully automated continuous manufacturing platform, features 500 L single-use perfusion bioreactors single-use perfusion bioreactors capable of high-density cultures while all downstream process steps are connected and handled by SymphonX™, a fully automated and highly customizable purification platform.

Agility with Demonstrated Gains
MaruX leverages the agility of single-use facilities with the flexibility to deliver fluctuating commercial demand requirements. It is suitable for converting fed-batch processes to continuous, while adaptable for transfer of other integrated biomanufacturing processes for high cell densities.
- Flexibility throughout product lifecycle to cope with fluctuations in demand forecasts
- Demonstrated productivity gains over fed-batch
- Equivalent process development cost and timeline compared to fed-batch
- Potential for lower manufacturing and consumable costs

SymphonX™: One Rig to Run All Downstream Steps
A fully automated and highly customizable technology for end-to-end multi-functional downstream purification, providing the connectivity, integration, advanced automation and buffer management needed to realize the high-productivity promise of continuous processing. Designed to simplify and de-risk operations, SymphonX operates over a range of scales, offering:
- Productivity improvements through process intensification
- Regulatory risk reduction
- Sustainable workflows
Our experts have set new industry expectations and raised the bar on what is achievable with continuous bioprocessing, with long duration runs at a clinically and commercially relevant manufacturing scale. Highlights include:

Successful 40-day Manufacturing Scale Perfusion Run
- After 10 days of growth the target of 120×106 cell density was maintained for 30 days.
- Average titer of 1.9 g/L/day
- Titer yield between bioreactor and permeate was >90% throughout the duration of the culture
- Product quality was equal and improved in some instances when compared to fed-batch cultures
- Total yield >30 kg of mAb

Continuously Innovative
We continue to push the boundaries of scale and culture cell density in our manufacturing process through innovation. By embracing continuous bioprocessing, we aim to streamline production, ensure timely and cost-effective delivery of biologics and improve patient access to life-changing medicines.
- Perfusion-ready cell line development and early process development available immediately
- 50 L demonstration and 500 L GMP MaruX coming soon