Transforming the Biopharmaceutical Industry: Addressing Patient Access to Medicines with Interconnected Networks
By Francisco Gonzalez, Associate Director of Strategic Technical Marketing and Lisa Emily, Director of Corporate Communications
Source: FUJIFILM Diosynth Biotechnologies
The global biopharmaceutical industry continues to exhibit resilience over the past several years, given the fact that it faced challenges during the global COVID-19 pandemic. The pervasive challenges caused development delays, significant economic pressure, operational constraints, and overall supply chain disruptions. In fact, the biopharmaceuticals’ market size is estimated over $516 billion in 2024, and it is projected to reach approximately $800 billion by 2030, growing at a CAGR of around 8% during the forecast period. (1, 2) However, even with the challenges related to the pandemic, the biopharmaceutical industry in particular continued to make treatments more accessible for all patients, persevered by adopting more efficient processes given tight timelines, strengthened supply chain with continuous improvement strategies, and sustained expected high quality standards, meeting compliance requirements.
Within the contract development and manufacturing organization (CDMO) space of biopharmaceuticals, CDMOs have focused on ways to overcome challenges customers face to meet their needs more effectively. Some of the improvements are as follows:
- Providing capacity and availability to meet market demands with access to diverse manufacturing strategies,
- Minimizing the involvement of multiple parties within the product life cycle supported by efficient technology transfer and on-time delivery,
- Providing access to a depth of knowledge and scientific experience to maximize the benefits of innovation and development.
What the customers are saying
Customers prefer to partner with a CDMO that can support them through the entire product life cycle with transparency and trust. They need a partner who can navigate with experience the hurdles of process development and manufacturing, demonstrating excellence, accuracy and precision with supporting controls required for safety and high-quality products. All of which are key to bring customers’ medicines from pre-clinical to clinical to commercial manufacturing successfully.
A reliable and experienced CDMO partner should guarantee strong technical support and the capacity needed at the appropriate scales and locations. Additionally, the appropriate partner should be able to demonstrate the ability to maintain a molecule’s performance with a high degree of accuracy, meeting regulatory requirements. Providing access to a well-established manufacturing network removes the risk of multiple tech transfers and third parties needed to achieve scale, cost minimization, and right-first-time delivery from optimum locations.
Listening to customers and market demands: FUJIFILM Diosynth Biotechnologies’ strategy and KojoX™
CDMOs that are well-positioned to respond to customers’ demands and market trends can adjust their business models and strategy to adapt and succeed. FUJIFILM Diosynth Biotechnologies (FDB) recently shared its Partners for Life strategy focused on transforming the customer experience – using trust as the cornerstone of that effort.3 This means that FDB is moving beyond the traditional CDMO transactional business model to provide a holistic approach to customers’ entire pipelines – from cell line development to commercial production – across its global network. The strategy focuses on three main pillars: People First, Transforming the Industry, and Unprecedented Delivery.3
Transforming the industry requires an intentional, comprehensive, paradigm shift to the approaches for facility layout and design, equipment, procedures and processes. FUJIFILM Diosynth Biotechnologies has launched its KojoX™ operating philosophy – its solution to transforming the biopharmaceutical industry.
In Japanese “Kojo” has dual meaning – “factory” and “improvement”. KojoX™ is a uniform operating philosophy, or an operational ecosystem, that when applied across sites, scales and modalities, harmonizes facility layout and design, equipment, processes and procedures through modular concepts. This approach is intended to help their customers’ molecules progress through the regulatory process quickly and efficiently to improve patient access to medicines.
Flexible production capacity offers the ability to optimize using a combination of single-use and/or stainless-steel technologies where separate lanes can be combined both in size and type providing a future-proof infrastructure that can allow flexible capacities for different modalities. The illustration below provides an outline of the KojoX ecosystem where its single-use, hybrid and stainless-steel networks are integrated, including the ability to multiplex, providing access to capacity throughout the product life cycle regardless of scale or location.
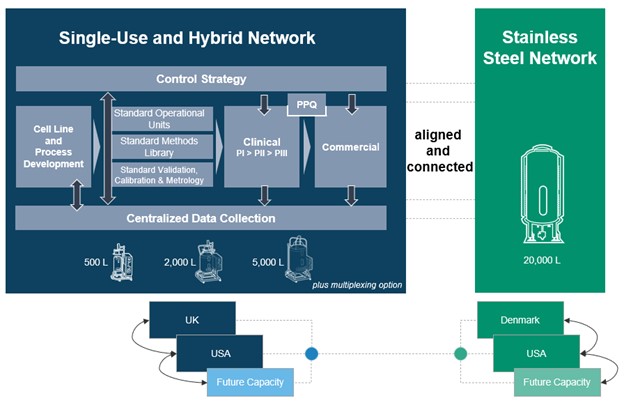
KojoX ecosystem: single-use, hybrid network (UK and USA) integrated with stainless-steel network (Denmark and USA). Single-use network includes bioreactor volumes from 500 to 5,000 L that can be multiplexed allowing for scale-up and scale-out manufacturing strategies that could feed into large scale 20,000 L production. The stainless-steel network is designed to host 20,000 L bioreactors as a scale-up strategy. This approach provides access to capacity throughout the product life cycle, from product development to clinical phases to commercialization, regardless of scale or location, with control strategy and centralized data collection systems to support production.
KojoX builds on modularity across three main functional areas: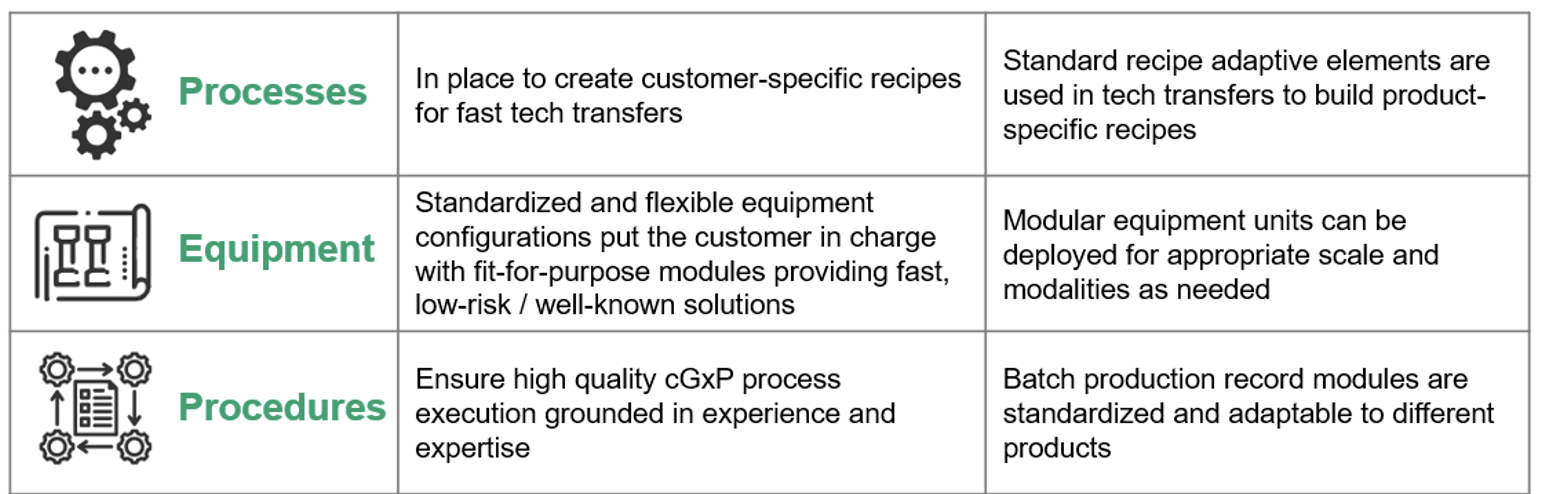
Introducing the first KojoX flexible single-use cell culture facility in the UK
FUJIFILM Diosynth Biotechnologies selected the capital investment project previously announced for the Billingham, UK, site for the first application of the KojoX operational model in the single-use network to meet customers’ demands.4 This flexible single-use cell culture facility is scheduled to be operational in 2026.5
A significant project scope change focused on applying the KojoX modular design concepts, allowing lanes to mix 2,000 and 5,000-liter bioreactors. This innovative design ensures flexibility while future-proofing infrastructure for rapid capacity additions as demand dictates. The Billingham facility represents a strategic move towards adaptability and responsiveness in the ever-evolving biopharmaceutical industry. The KojoX design aspires to reduce tech transfer times into manufacturing by approximately 40%. It will also allow for programs to be sited anywhere in the FUJIFILM Diosynth Biotechnologies KojoX network without the need for further tech transfers.
FDB’ strategic capital investments, guided by the KojoX operational framework, underscore the company’s commitment to innovations to improve efficiency and flexibility through modular design principles, paving the way for groundbreaking advancements for improved patient access to medicines.

FUJIFILM Diosynth Biotechnologies’ single-use cell culture facility in Billingham, UK, applying KojoX philosophy with modules of 2,000 L and 5,000 L single-use bioreactors for mammalian cell culture designed to feed into large scale 20,000 L production.
Finally, this flexible, modular design provides adaptability to scale out and scale up for rapidly changing market demands, providing access to multiple manufacturing strategies. When applied across FDB’s network, it is expected to improve time-to-market, supply resilience, and geographic diversity of supply, aiming to provide fast access to localized manufacturing capacity.
Accessing a depth of knowledge and scientific experience – leads to innovation and development
Customers should ensure their CDMO partners not only have the infrastructure to support their programs, but also that they have the skilled scientific staff to move their programs forward. To successfully transfer scientific and technical data from the customers to the program development and design team, customers need access to the right combination of team members to manage program milestones, encourage customer engagement through thorough, formalized communications systems, and the ability to cultivate a culture of trust that culminates in efficient and cost-effective program delivery.
A reliable partner will maintain communication to discuss program updates with you. Fostering effective touchpoints will result in beneficial adjustments that will impact the safety, quality, timeline, and cost of your program. That partner should be able to guarantee that your program advances seamlessly from beginning to end, including all necessary quality checks. They will also have resources in place to mitigate potential supply chain issues resulting from the use of third parties, avoiding costly delays that could impact patients’ access to medicines.
FUJIFILM Diosynth Biotechnologies has over thirty years of experience in developing and manufacturing drug substance of recombinant proteins, monoclonal antibodies, vaccines, among other large molecules, viral products and medical countermeasures expressed in a wide array of microbial, mammalian, and host/virus systems. Additionally, their drug product offering is supported by a leadership team with over 75 years of combined experience, and they have been successfully navigating clinical and commercial biologics finished goods manufacturing for over 15 years.
At FUJIFILM Diosynth Biotechnologies our systems ensure right-first-time delivery and offer the flexibility to leverage our extensive experience and end-to-end value chain as needed. As we develop dependable relationships with our clients, we continue to prioritize the people who matter most: patients.
References
- Biopharmaceutical Industry Size & Share Analysis – Growth Trends & Forecasts (2024 – 2029). Mordor Intelligence.
https://www.mordorintelligence.com/industry-reports/global-biopharmaceuticals-market-industry - Biopharmaceuticals Market Size, Share, Growth, COVID-19 Impact & Industry Analysis, By Type, By Application, By Distribution Channel, and Regional Forecast, 2023-2030. Fortune Business Insights. (2023, November). https://www.fortunebusinessinsights.com/biopharmaceuticals-market-106928
- FUJIFILM Diosynth Biotechnologies Partners for Life Strategy 2024. (2024, March 14). https://view.ceros.com/fujifilm-diosynth/partners-for-life-strategy-2024/p/1
- $850 Million USD (¥90 Billion Yen) investment in FUJIFILM Diosynth Biotechnologies to add additional development and manufacturing capacity. (2021, June 29). https://fujifilmdiosynth.com/about-us/press-releases/850-million-usd-%c2%a590-billion-yen-investment-in-fujifilm-diosynth-biotechnologies-to-add-additional-development-and-manufacturing-capacity/
- Introducing the 1st KojoX™ Flexible Cell Culture Facility – in the UK. FUJIFILM Diosynth Biotechnologies. (2024, March 15) https://view.ceros.com/fujifilm-diosynth/flexible-cell-culture-facility/p/1
About the Authors
Francisco Gonzalez is Associate Director of Strategic Technical Marketing at FUJIFILM Diosynth Biotechnologies, responsible for supporting and leading marketing activities for all service offerings with focus on Drug Product, Finished Goods, and CMC/Analytical services. He has been with FDB for over 4 years where he started as a member of the Formulation Development team at RTP, NC. He earned a Licentiate in Chemistry from Universidad Simón Bolívar in Venezuela and a Ph.D. in Chemistry and Biochemistry from Baylor University. He continued as a post-doctoral fellow at the University of South Carolina with emphasis in protein aggregation in Alzheimer’s disease. With over 25 years of scientific experience, he has worked in research, product and process development, product applications, validation and continuous improvement for the Venezuelan Oil Company, Procter & Gamble, Selah Genomics, Patheon-Thermo Fisher, Ethicon-Johnson & Johnson and Unchained Labs.
Lisa Emily is Director of Corporate Communications at FUJIFILM Diosynth Biotechnologies, responsible for developing, managing and applying corporate communication guidelines for social media, marketing communications and crisis communications. She joined FUJIFILM Diosynth Biotechnologies in 2021, after working with a sister Fujifilm subsidiary for over 25 years in Corporate Communications. Lisa earned her B.S. in Business Administration with a minor in Linguistics from Lander University in 1984. Lisa worked in county government for over 7 years, gaining valuable government affairs experience.