
Process Validation
PROCESS VALIDATION – DEFINING A CAPABLE MANUFACTURING PROCESS
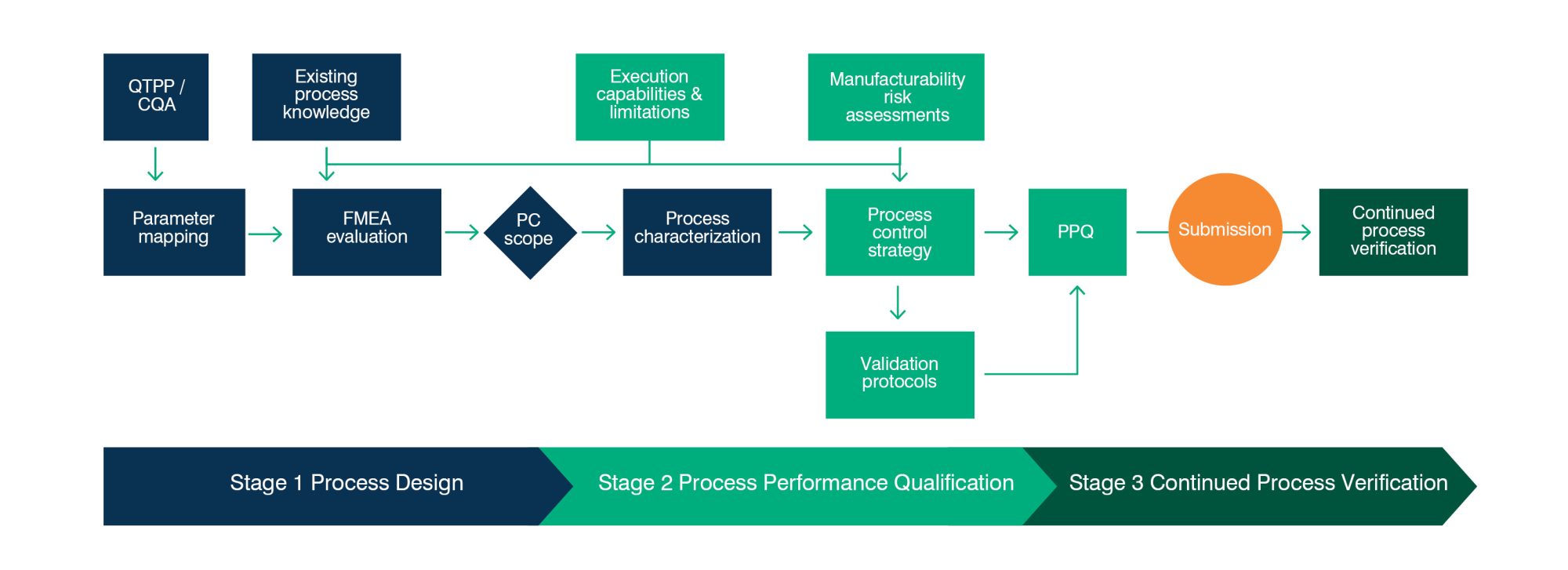
Process Validation (PV) is the process of collecting and evaluating process data and knowledge to establish a robust and controlled process, capable of routine commercial manufacture that consistently delivers product that meets the required specification.
Our flexible and proven systems are adaptable to our clients’ needs and their approach to PV. We work with you from initial PV Design (Stage 1), through Process Performance Qualification (PPQ) (Stage 2), Submission and Continued Process Verification (Stage 3). Our support is maintained through inspection processes all the way to long-term production.
Our network has 19 commercial licences for biologics from mammalian and microbial expression systems, the most recent received in 2022.
FDB has tried and tested systems and tools in place to establish process control strategies leading to successful PPQ, inspection and approval.
Contact us to learn how our CMC support services can advance your therapies